1. Background
Hepatitis C virus (HCV) infection is prevalent at alarming levels, affecting more than 70 million people globally (1). Chronic hepatitis C, hepatocellular carcinoma, and liver cirrhosis mostly result from HCV infection, and these may lead to liver transplantation or even death (2, 3). Annually, 3 - 4 million individuals are infected with HCV, and over 350,000 people die due to HCV-related hepatic diseases (3).
New oral antiviral agents have emerged with better safety and efficacy profiles than the interferon (IFN)-based regimen traditionally used against HCV infection (4). The second generation of direct-acting antivirals (DAAs) in combination with or without ribavirin is highly effective with fewer side effects, particularly among patients having genotype 1 infection who are difficult to treat with IFN-based regimen (5). Moreover, HCV genotype 4 is more challenging to treat with sustained virologic response (SVR) rates between 32% - 55% when treated with IFN and ribavirin (6).
One of the first DAAs, telaprevir, combined with ribavirin and pegylated IFN, resulted in better clinical outcomes in patients with HCV genotype 4 than telaprevir monotherapy or ribavirin and pegylated IFN alone (7, 8). However, this treatment strategy included a lengthy treatment process with side effects. More recently, the second-generation DAAs have shown better results with shorter 12-weeks treatment period for HCV genotype 4 infection without the requirement for IFN (9). In January 2016, a combination treatment of DAAs, grazoprevir, and elbasvir was approved by the US Food and Drug Administration (FDA). Using this combination, 88.9% patients with HCV genotype 4 infection who received the treatment achieved SVR at 12 weeks follow-up (SVR12) when they had a combination with or without ribavirin (10).
Kidney transplant (KT) recipients are at risk for HCV infection with a rate of ~5% in high-income countries and significantly increased rates in low-income countries (11, 12). Those who receive KT are subjected to an immunosuppressed state, which could significantly increase the infection risk and precipitate disease progression (13). The hepatic failure risk is the chief issue, as it is the fourth most common reason for death (8% - 28%) in patients who survived for many years post-KT (14). Besides, HCV has a potential influence on the survival and persistence of the transplanted graft (15).
Indeed, the existing evidence indicates that long-term survival (of patients and grafts) in KT patients who are HCV-positive is considerably lower than that of patients who are HCV-negative (11). Thus, preventing and managing HCV infection is a critical issue in KT therapy and outcome.
Traditionally, the use of IFN and ribavirin post-KT carried an increased risk of graft loss due to rejection (16). However, DAAs have shown the potential to achieve SVR in this patient population and improve graft and patient outcomes (15). Still, the data on the use of DAAs post-KT in the patient population with HCV genotype 4 infection is limited.
2. Objectives
This study aimed to evaluate the effectiveness of grazoprevir/elbasvir combination without ribavirin in post-KT patients with HCV genotype 4 infection and the occurrence of any adverse events.
3. Methods
3.1. Study Design
This study was a case series study conducted at King Faisal Specialist Hospital and Research Center (KFSHRC), Riyadh, Saudi Arabia, between January and December 2018, and followed up for 12 months. This study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review board of King Faisal Specialist Hospital and Research Center (IRB no.: 2171009).
3.2. Patients
Post-KT patients with chronic HCV genotype 4 infection were enrolled in the study. Patients were included in the study if they fulfilled the inclusion criteria. The eligibility criteria are listed in Table 1. All patients were treatment naive.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Adults (aged 18 years and above) | Patients under the age of legal consent, patients who were mentally or legally incapacitated, patients with significant emotional problems at the time of pre-study screening visit or anticipated during the conduct of the study, or patients with a history of clinically significant psychiatric disorder, which in the opinion of the investigator, would interfere with the study procedures |
| Based on creatinine clearance (CrCl), patients with stable graft function after KT were categorized into two arms: < 30 or ≥ 30 mL/min | Patients infected with HCV genotypes 1, 2, 3, 5, or 6, except those with genotype 1 or 6 mixed with genotype 4 infection |
| Patients infected with HCV after KT (positive HCV RNA with acknowledged HCV genotype 4 (comprising patients with mixed infections: genotypes 4 and 1 or 4 and 6) | Patients co-infected with HBV or HIV |
| Patients showing evidence of chronic HCV infection confirmed by a liver biopsy performed before the baseline | Patients with confirmed decompensated liver disease and having or had ascites, esophageal or gastric variceal bleeding, hepatic encephalopathy, or other indications of advanced liver diseases |
| Patients who agreed to take part in the study by providing written informed consent having gained knowledge on study details, other therapy choices available, risks of participation | Pregnant or breastfeeding women or male patients with a pregnant partner |
| Patients with contraindications for grazoprevir/elbasvir | |
| Patients who are or have been involved in a trial and received an experimental drug within 1 month of enrolling the study and was unwilling to abstain from participating in another similar study while being a part of the current study | |
| Patients with abnormal laboratory or electrocardiogram findings or an illness that could affect the study or induce a harm to patients |
3.3. Interventions
All patients received a fixed-dose combination of grazoprevir/elbasvir (Zepatier 50 mg/100 mg) without ribavirin once daily for 12 weeks after KT. The treating physicians made all the decisions regarding medical management based on the patients’ clinical status. Drugs, which could severely or moderately induce cytochrome P450 (CYP) 3A4 and P-glycoprotein (P-gp), such as phenobarbital, nafcillin, phenytoin, rifampin, carbamazepine, and St. John’s Wort (Hypericum perforatum, a herbal remedy), were stopped during the study period due to their known contraindications with certain DAA therapies. Any such concomitant medications/therapies discontinued during the study period were restarted two weeks post-last study drug dose and continued during the follow-up period. The common drugs used for immunosuppression, such as tacrolimus, mycophenolate mofetil, and prednisone, were allowed to prevent donor organ rejection.
3.4. Clinical Data Collection and Outcomes
Clinical data were collected using a standardized data collection form through direct patient interviews, integrated clinical information systems, or electronic medication administration records and charts review. The standardized definitions of all patient-related variables and clinical diagnoses were used.
Serum samples were collected at the following intervals: baseline, weeks 1, 2, 4, 6, 8, 12, 24, and 36, and the HCV RNA in serum was determined by real-time polymerase chain reaction (RT-PCR).
HCV detection and quantitation was performed using Abbott RealTime M2000 instrument. The assay utilizes two distinct sets of primers and probes. The first set targets a conserved region of the 5’ untranslated region of the HCV genome. The second set is specific for internal control (IC) that is processed with each sample to control for sample recovery and inhibition. The Abbott RealTime HCV assay provides detection limit (analytical measurement range) from 30 to 100,000,000 IU/mL.
Liver function test, complete blood count, including hemoglobin during and after therapy, follow-up assessments for any changes from baseline, blood chemistry, and other laboratory tests were collected whenever available or requested by the treating physician.
The primary endpoint was the effectiveness of grazoprevir/elbasvir combination without ribavirin in post-KT patients with HCV genotype 4, which was defined by the end of treatment response (ETR) marking the therapy completion. Secondary endpoints included the proportion of patients who achieved SVR at 12 weeks (SVR12) posttherapy discontinuation, the kinetics of circulating HCV RNA post-treatment discontinuation, renal function determined using estimated glomerular filtration rate (eGFR) and albumin-creatinine ratio, and the rate of acute rejection.
3.5. Definitions and Follow-up
Effectiveness was defined at various time intervals during the trial period. The specific endpoints were HCV RNA less than the lower limit of quantitation (HCV RNA < LLOQ) at weeks 4/8 and 12 of treatment, defined as HCV clearance and ETR, respectively, and 12 weeks follow-up after treatment (SVR12).
Nonresponse was defined by HCV RNA value not lesser than the lower limit of quantitation (or absence of HCV RNA < LLOQ).
A virologic breakthrough was defined as HCV RNA ≥ LLOQ after being lower than LLOQ earlier during treatment. Confirmation was defined by HCV RNA ≥ LLOQ in another blood sample collected within two weeks.
Relapse was defined by confirmed HCV RNA ≥ LLOQ after the termination of trial drug but being undetectable after therapy completion. Relapse confirmation was defined by HCV RNA ≥ LLOQ from another blood sample collected within two weeks.
The safety and tolerability of the study medications, including all adverse events, were recorded from initiating the therapy until 180 days after completing it. The worsening of a pre-existing condition, associated with the timing of the use of grazoprevir/elbasvir, was also classed as an adverse event.
Concomitant medications or vaccinations received within 30 days of starting grazoprevir/elbasvir and up to four weeks after discontinuation of the study treatment were recorded. In case of a clinical indication observed for any drug or vaccine forbidden overtly during the trial period, the study drug was discontinued. Nevertheless, all key stakeholders, such as the investigator, sponsor, and patients, took part in the decision-making process of continuing the study drug or vaccination schedule.
3.6. Data Analysis
Data analysis was performed using SPSS version 21 (SPSS IBM, New York, USA). The data are presented as median and range or percentages. Patients who were administered at least a single dose of grazoprevir/elbasvir combination (Zepatier 50 mg/100 mg) without ribavirin were included in the study analysis.
4. Results
4.1. Baseline Data
Nine post-KT patients with HCV genotype 4 infection who fulfilled the eligibility criteria were recruited for this study. The median time to starting treatment following KT was 72 months in our study population. All the patients received a fixed dose of grazoprevir/elbasvir post-KT. There were four male and five female patients, with a median age of 44 (28 - 64) years. Three (33%) patients had mixed genotypes 4 and 1. All the patients were naïve to therapy and had a stable renal function. Two patients had fibrosis score 0 (22%), three patients had fibrosis score 1 (33%), one patient had fibrosis score 2 - 3 (11%), and three patients had fibrosis score 4 (33%). Only one patient in the study had a baseline creatinine clearance of < 30 mL/min. The baseline data for all nine patients are presented in Table 2.
| Patient Characteristics | Value, Median (Range) |
|---|---|
| Age, y | 44 (28 - 64) |
| Gender, n | |
| Men | 4 |
| Women | 5 |
| Liver fibrosis score | |
| 0, n | 2 |
| 1, n | 3 |
| 2 - 3, n | 1 |
| 4, n | 3 |
| HCV genotype | |
| 1 and 4, n | 3 |
| 4, n | 6 |
| HCV RNA pretreatment: viral load, log10 IU/L | 5.89 (5.21 - 6.66) |
| Hemoglobin, g/L | 124 (10 - 157) |
| Bilirubin, µmol/L | 8 (3 - 13) |
| ALT, U/L | 24 (10.0 - 47.0) |
| AST, U/L | 21 (8.0 - 30.0) |
| Albumin, g/L | 43 (34.1 - 46.0) |
| Platelet count, 109/L | 259 (144 - 346) |
| Creatinine, µmol/L | 93 (64 - 400) |
| International normalized ratio | 1 (0.9 - 1.1) |
| eGFR, ml/min | 60 (14 - 60) |
| Sodium, mmol/L | 140 (137 - 144) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; HCV, hepatitis C virus.
4.2. Virologic Response
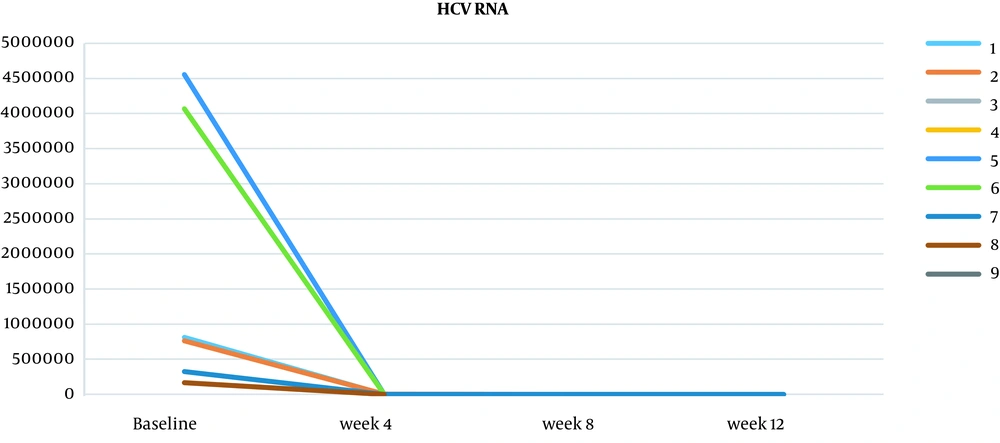
Viral load was measured at baseline and then at 4, 8, and 12 weeks. At four weeks, two patients achieved HCV clearance, and there was a reduction in serum HCV RNA levels in all the patients who were tested (7/9 patients were examined at week 4, and two were not) (Table 3 and Figure 1). After eight weeks, of the six patients tested, HCV clearance was achieved in an additional four patients (three patients were not examined at week 8). By week 12, HCV RNA was measured, and all the patients achieved ETR, and subsequently, SVR12 was confirmed in all the patients, suggesting that the 12-week combination therapy of grazoprevir/elbasvir was sufficient to induce SVR in this patient population.
| Case | Post-treatment Viral Load Status | SVR12 | ||
|---|---|---|---|---|
| Week 4 | Week 8 | Week 12 | ||
| 1 | < 30 IU/mL | HCV clearance achieved | ETR achieved | Achieved |
| 2 | 440 IU/mL | HCV clearance achieved | ETR achieved | Achieved |
| 3 | N/A | ETR achieved | Achieved | |
| 4 | N/A | < 30 IU/mL | ETR achieved | Achieved |
| 5 | Undetectable | HCV clearance achieved | ETR achieved | Achieved |
| 6 | 161 IU/mL | N/A | ETR achieved | Achieved |
| 7 | < 30 IU/mL | HCV clearance achieved | ETR achieved | Achieved |
| 8 | Undetectable | N/A | ETR achieved | Achieved |
| 9 | < 30 IU/mL | HCV clearance achieved | ETR achieved | Achieved |
4.3. Secondary Endpoints
Renal function in all nine patients remained stable during and after the treatment period, with no deterioration of graft function. An improvement in alanine aminotransferase and aspartate aminotransferase levels was recorded from baseline to end of treatment. The changes in median clinical test values over the 12 weeks after treatment are presented in Table 4.
| Laboratory Values | Baseline | Post-treatment Changes | ||
|---|---|---|---|---|
| 4 Weeks | 8 Weeks | 12 Weeks | ||
| Hemoglobin, g/L | 124 (101 - 157) | 121 (78 - 155) | 121 (92 - 151) | 124 (80 - 152) |
| Bilirubin, µmol/L | 8 (3 - 13) | 7 (5 - 28) | 11 (4 - 24) | 9 (3 - 23) |
| ALT, U/L | 24 (10.0 - 47.0) | 11 (6 - 15) | 11 (6 - 17) | 9 (8 - 15) |
| AST, U/L | 21 (8.0 - 30.0) | 12 (8 - 15) | 12 (10 - 17) | 11 (7 - 16) |
| Albumin, g/L | 43 (34.1 - 46.0) | 43 (24 - 44) | 42 (33 - 45) | 43 (37 - 46) |
| Platelets, 109/L | 259 (144 - 346) | 231 (139 - 290) | 255 (132 - 344) | 183 (136 - 318) |
| Creatinine, µmol/L | 93 (64 - 400) | 89 (64 - 428) | 86 (73 - 108) | 96 (65 - 644) |
| International normalized ratio | 1 (0.9 - 1.1) | 1 (0.9 - 1.1) | 1.1 (1 - 1.1) | 1.1 (1 -1.2) |
| eGFR, ml/min | 60 (14 - 60) | 60 (12 - 60) | 60 (54 - 60) | 60 (8 - 60) |
| Sodium, mmol/L | 140 (137 - 144) | 139 (136 - 142) | 139 (137 - 141) | 140 (133 - 141) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate.
aValues are expressed as median (range).
4.4. Immunosuppressive Therapy and Safety of Interventional Therapy
All the patients were on tacrolimus as the main immunosuppression medication, except for one who was on cyclosporin. The once-daily dose of grazoprevir/elbasvir was well-tolerated in all nine patients. No severe adverse events were reported. No patients discontinued the study due to treatment therapy, indicating the safety of grazoprevir/elbasvir in this patient population.
5. Discussion
This study aimed to evaluate the effectiveness and the occurrence of adverse events with grazoprevir/elbasvir combination treatment without ribavirin in post-KT patients with HCV genotype 4 infection. A cohort of nine patients achieved SVR12 with no adverse events during the treatment period, and renal function remained stable during and after treatment. Overall, the results of this small study indicate that grazoprevir/elbasvir combination treatment without ribavirin was effective and safe for this series of post-KT patients with HCV genotype 4 infection.
Previously, the treatment of HCV infection after KT was tested on populations where most patients had genotype 1 infection. As genotype 4 is less common, data on treating this population of patients is limited. However, genotype 4 is the dominant HCV genotype in the Middle East and North Africa and globally accounts for around 20% of all HCV infections (17, 18). Thus, studies that test the efficacy and safety of current treatment regimens for HCV are warranted for this patient population.
Current recommendations from the American Association for the Study of Liver Diseases (AASLD) and Infectious Diseases Society of America (IDSA) for HCV genotype 4 patient population without liver cirrhosis include a daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) for 12 weeks, as one of the four treatment strategies (https://www.hcvguidelines.org). This guidance is based on high-level evidence, which tested the efficacy of this treatment.
SVR12 was found to have been achieved in all the patients with HCV genotype 4 infection, and 96% of the patients with HIV co-infection had achieved SVR12. Cirrhosis, baseline resistance-associated substitutions (RASs), and genotype 4 subtypes presented in some patients did not appear to impact SVR12 rates (19-21). However, for HCV-positive patients post-RT, the AASLD-IDSA guidelines urge caution with this approach because of drug interactions with immunosuppressants such as calcineurin inhibitors. Cyclosporin treatment is not recommended in combination with grazoprevir and elbasvir, as data suggests a 15-fold increase in grazoprevir area under the curve (AUC) and a 2-fold increase in elbasvir AUC (22). Tacrolimus is considered better tolerated, and anticipation of rise in tacrolimus levels of 40% - 50% when administered along with grazoprevir is proposed, with no alterations in dosage expected.
Nevertheless, tacrolimus levels should be monitored throughout the study period. Therefore, tacrolimus is only recommended as an alternative treatment for patients post-KT with HCV genotype 1 or 4 without baseline NS5A RASs with elbasvir for 12 weeks (https://www.hcvguidelines.org). The results of this study, where the standard immunosuppression included tacrolimus, show that SVR12 can be achieved in 100% of patients, suggesting that grazoprevir/elbasvir is worth considering after KT.
The use of grazoprevir/elbasvir after KT is limited to relatively small populations, but the high rates of SVR12 seen in this study and others are encouraging. In 11 patients who received KT and had significant abnormal renal function (GFR < 40 mL/min), 12 - 16 weeks of treatment with grazoprevir/elbasvir showed an SVR12 of 100%. Of the 11 patients studied, one patient had HCV genotype 4 infection, and the patient exhibited a virologic response within 8 weeks and SVR at 12 weeks (23). Grazoprevir/elbasvir has also proven effective in 20 HCV-negative KT patients with organs transplanted from genotype 1 HCV RNA-positive donors. All the patients achieved SVR12 after 12 weeks of grazoprevir/elbasvir treatment and 16 weeks with the introduction of ribavirin for patients with genotype 1a and baseline NS5A RASs (24). Interestingly, the 1-year follow-up showed that the patients who received HCV RNA-positive donor organs had better renal function than controls with organs transplanted from HCV-negative donors (25).
Another trial showed that HCV-negative KT patients with organs transplanted from HCV RNA-positive donors of all genotypes achieved SVR12 when prophylactic treatment of grazoprevir/elbasvir was administered before transplantation, with the addition of sofosbuvir for genotype 2 or 3-infected donor organs (26).
Despite the small study subpopulations, several studies have shown that other DAA combinations can effectively treat HCV genotype 4 post-KT. In 80 liver transplant and 20 KT patients with HCV genotype 1 - 6, including genotype 4, 98% achieved SVR12 after glecaprevir/pibrentasvir treatment. The safety profile was good, and most adverse events were mild (27). However, caution is advised as glecaprevir/pibrentasvir also has the potential for drug-drug interactions with cyclosporine and tacrolimus based on AASLD-IDSA guidelines. A combination of ledipasvir/sofosbuvir was evaluated in a randomized phase II trial in 114 KT recipients with genotype 1 or 4 infections. The drug combination was well tolerated with an acceptable safety profile, and all patients achieved SVR 12 (28).
Sofosbuvir-based regimens have been studied relatively widely in post-KT patients. The HCV-TARGET real-world data studied various regimens for 443 patients with genotype 1 or 3 infections and found that SVR12 was achieved in 94.6% of patients with KT and 90.9% in dual liver transplant and KT recipients; ribavirin use did not influence SVR12 (29). Sofosbuvir-based therapy is generally well tolerated in KT patients with HCV infection, with most patients achieving SVR12. Various sofosbuvir-based regimens show none or minimal apparent drug-drug interactions with calcineurin inhibitors; thus, dose adjustment of immunosuppressants is not needed (30-32).
Some DAAs such as simeprevir, ledipasvir, and ombitasvir/ritonavir-boosted paritaprevir have significant interactions with immunosuppressive agents (16). The need for calcineurin inhibitor adjustment and transient increase in serum creatinine is observed in some patients. Sometimes months after treatment completion, it might reflect enhanced metabolism of tacrolimus associated with the resolution of liver injury. Therefore, it is important to follow patients strictly, even after the treatment period (33).
The use of DAAs in patients with chronic kidney disease means that treatment can often be successful before the need for transplantation. Delaying therapy and providing treatment during the post-KT period is also an option that can be influenced by several factors, including patient preference, the extent of liver injury, the availability of a living or deceased donor, and the option of transplanting a kidney from an HCV-positive donor with a potentially short waiting time and expanded organ donor pool (34).
This study has some limitations. It is a small case series, so there was no randomization to treatment groups. A larger study from multiple centers would allow the comparison of grazoprevir/elbasvir combination without ribavirin treatment with standard treatment.
Despite the current study being a small single-center cohort with limited patient numbers and lacking a control or treatment comparator, it does confirm that grazoprevir/elbasvir combination without ribavirin is effective in achieving SVR12 in post-KT patients with HCV genotype 4 infection. In this case series, grazoprevir/elbasvir combination was a safe treatment option with no adverse events reported.