1. Context
The first case of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection was reported in China in December 2019 (1). The World Health Organization (WHO) declared Coronavirus Disease 2019 (COVID-19) a pandemic on March 11, 2020. As of October 19, 2020, > 48 million cases and > 1,200,000 deaths were reported (2). A more severe disease course and a higher mortality rate have been reported in elderly patients, as well as those with underlying conditions, including hypertension, asthma, diabetes mellitus, chronic lung disease, cardiovascular conditions, obesity, and chronic kidney disease (3-8). COVID-19 clinical presentations could vary greatly but often include respiratory, gastrointestinal, renal, and neurological manifestations (9, 10).
Recent studies have reported COVID-19 patients presenting with acute hepatitis before demonstrating respiratory symptoms (11). While our understanding of SARS-CoV-2 pathogenesis continues to grow, initial studies suggest that the virus could lead to liver injury mainly by binding to Angiotensin-Converting Enzyme 2 (ACE2) receptors on hepatocytes or causing an immune-mediated hepatic injury through cytokine storm activation (12-14). Several studies suggest that COVID-19 could lead to liver injuries and elevated Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), and total bilirubin, particularly among severe COVID-19 cases (e.g., those admitted to Intensive Care Unit [ICU]) (15-18). Abnormal liver functions among COVID-19 patients have also been associated with increased disease severity and risk of mortality (19, 20). A recent meta-analysis of a few studies on the hepatic manifestations of COVID-19 estimated the pooled prevalence of pre-existing chronic liver disease at 1.9%, pre-existing liver cirrhosis at 0.4%, HBV at 0.9%, and HCV at 0.3% (15). While insightful, these findings are limited, as they are only based on less than a handful of small sample-sized studies conducted in China and the USA during the early months of the pandemic (i.e., as late as April 2020) (15). These findings also contradict other earlier studies that reported a significantly higher prevalence of liver complications among COVID-19 patients in China, where 2-11% of COVID-19 patients had liver comorbidities and up to 54% had elevated AST and ALT levels (21).
COVID-19-related hepatic complications are of concern, particularly among people living with HCV, HBV, or HBV/HCV co-infection with pre-existing liver complications (e.g., cirrhosis, liver failure, hepatocellular carcinoma) (15, 21, 22). Considering that around 290 million and 71 million people are living with HBV and HCV, respectively (23, 24), the number of patients with SARS-CoV-2 and HBV or HCV co-infection is likely to increase. Therefore, improving our understanding of the hepatic manifestations and comorbidities among COVID-19 patients living with HBV, HCV, or both is of utmost importance in enhancing the care provided for this large at-risk population.
2. Objectives
In this systematic review, we aimed to review and summarize the existing literature on COVID-19 patients living with HBV or HCV comorbidities. The findings of our review could provide information on clinical care and treatment options for COVID-19 patients with these co-infections.
3. Data Sources
The inclusion criteria and analytical plan were conceptualized a priori and are documented in the Open Science Framework ( https://osf.io/p4w3j/).
Following the Systematic Reviews and Meta-Analyses (PRISMA) and Peer Review of Electronic Search Strategies (25) guidelines (see Supplementary File S1 for PRISMA checklist), we searched PubMed, Scopus, Web of Science, CINAHL, Embase, and Google Scholar, as well as preprint databases, including medRxiv and bioRxiv from December 1, 2019, to August 9, 2020. Search terms were combined using appropriate Boolean operators and included subject heading terms/keywords relevant to COVID-19 (e.g., SARS-CoV-2 or coronavirus disease 2019 or COVID-19 or severe acute respiratory syndrome coronavirus 2 or coronavirus infection) and hepatitis B or C (e.g., liver fibrosis or liver cirrhosis or hepatic transplantation or liver transplant or hepatitis C or hepatitis B or HBV or HCV). Please see Supplementary File S2 for our sample search strategy.
4. Study Selection
Quantitative studies of any type (i.e., case report, case series, cross-sectional, case-control, cohort, and clinical trial) that reported individual-level and/or aggregate-level data on COVID-19 patients living with HBV, HCV, or HBV-HCV-co-infection were included in this review. Studies that combined the samples of HBV/HCV-positive and HBV/HCV-negative patients were only included if they provided subgroup analyses for people living with HBV, HCV, or HBV-HCV-co-infection. Studies were excluded if they did not present original empirical data or did not report any clinical data for patients. Two reviewers (HM and MV) independently screened all titles, abstracts, and full-texts. Duplicate records were excluded, and disagreements were resolved by discussion or arbitration by the senior author (MK).
5. Data Extraction
Data were extracted on a) Study characteristics, including first author, study period, study design, sample size, data type, and study population, b) Sociodemographic characteristics, such as patients' age and sex/gender, c) HBV- and HCV-related characteristics, including cirrhosis status, liver transplantation, received immunosuppressive therapy, and liver enzyme levels (e.g., AST, ALT), d) COVID-19-related characteristics, including symptoms and severity of COVID-19 defined as mild (i.e., no or mild pneumonia), severe (i.e., blood oxygen saturation ≤ 93%, dyspnea, or lung infiltrates > 50% within two days), or critical (i.e., septic shock, respiratory failure, or multiple organ failures) (26). Hospitalization, ICU admission, survival status (recovery or death), and non-hepatic-related comorbidities (e.g., hypertension, diabetes, cardiovascular diseases, Chronic Obstructive Pulmonary Disease [COPD], and malignancies) were also recorded.
5.1. Quality Assessment
Two authors (HM and MV) completed quality assessments independently, using the Joanna Briggs Institute critical appraisal tools (27). These tools assess different items (e.g., selection bias, information bias, and confounding bias) for various study designs, with nine items for case reports, 10 for case series, nine for cross-sectional studies, and 11 for cohort studies.
5.2. Statistical Analysis
Descriptive analyses were used for reporting the results. Continuous variables were summarized as mean and Standard Deviation (SD). Categorical variables were summarized by frequencies and percentages. Differences in continuous and categorical variables were compared using the two-tailed student's t-test and Fisher's exact test, respectively. The P values of less than 0.05 were considered statistically significant. For combining data from studies that reported aggregate-level data with those reporting individual-level data, we weighted the aggregate-level data by the number of patients. The proportion of death among reported patients was also measured and reported. The denominator and nominator for this measure were based on patients with HBV or HCV whose COVID-19 was diagnosed and reported. The main findings were stratified by study design. We also conducted a subgroup analysis by patients' receipt of immunosuppressive therapy.
6. Results
6.1. Study Characteristics
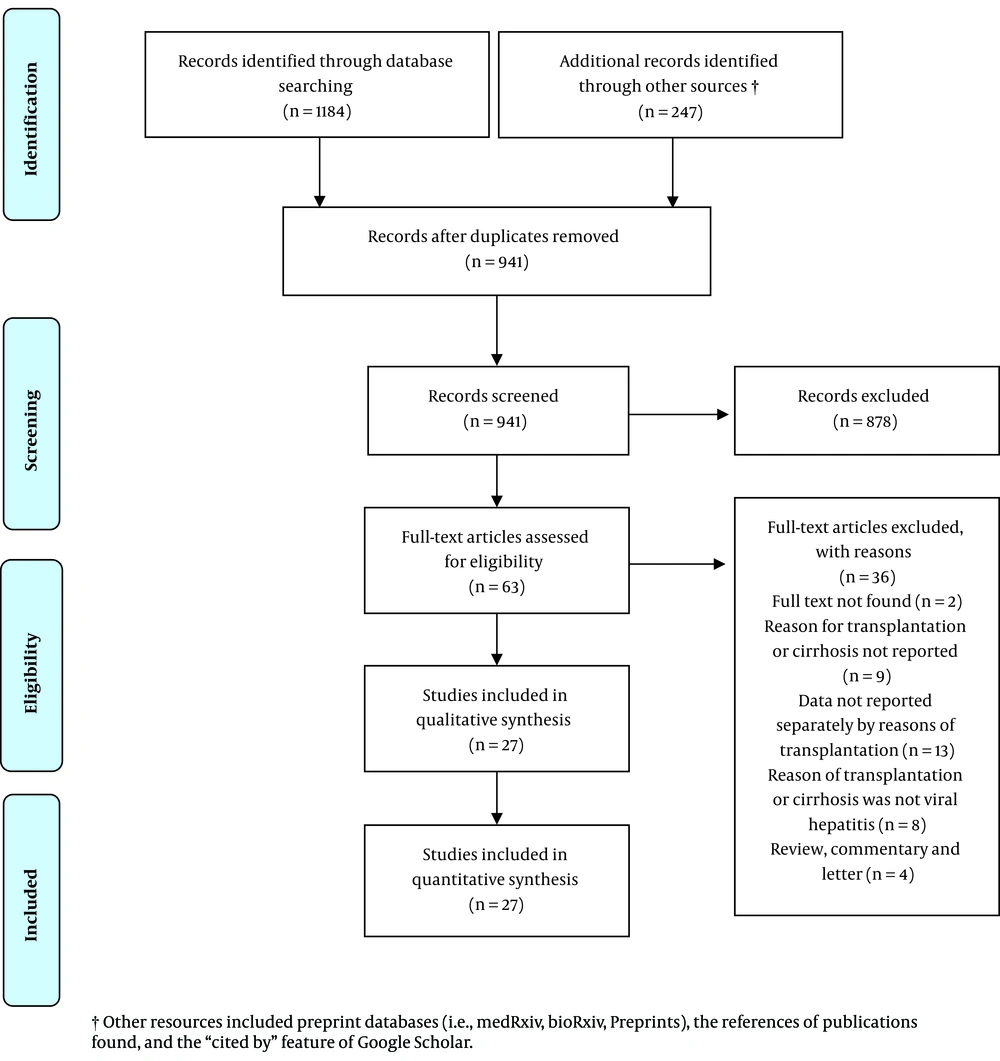
Out of the 941 uniquely identified records, 27 studies met the inclusion criteria and were considered for data extraction. Figure 1 shows the PRISMA flow diagram, and Table 1 presents the study characteristics. Of the 27 included studies with data on SARS-CoV-2 and HBV and/or HCV co-infection, 14 studies reported SARS-CoV-2 and HBV co-infection, 10 studies reported SARS-CoV-2 and HCV co-infection, and three reported both types of co-infection. Regarding their geographical locations, 14 studies reported data from China, three from the USA, two from Spain, two from Brazil, and the remaining six studies from Italy, Austria, Switzerland, the UK, Lithuania, and the UAE (one study each). All of the included studies were observational: 11 studies were case reports, 10 were case series, and six were cross-sectional. The majority of the included studies (n = 21) reported individual-level data, and six studies reported aggregate-level data.
| First Author | Setting | Study Perioda | Study Design | Data Type | Sample Size | Type of Hepatitis | Study Population | Quality Assessment |
|---|---|---|---|---|---|---|---|---|
| Aldhaleei (28) | UAE | Not reported | Case report | Individual | 1 | HBV | HBV patient | 7/8 |
| Chen (29) | China | Jan 5 to Feb 20 | Cross-sectional | Aggregate | 15 | HBV | HBV patients | 9/9 |
| Chen (30) | China | Jan 20 to Feb 29 | Cross sectional | Aggregate | 20 | HBV | HBV patients | 8/9 |
| De Gottardi (31) | Switzerland | Mar 4 to Mar 14 | Case report | Individual | 1 | HCV | LT recipient | 7/8 |
| Fernández (32) | Spain | Mar 5 to Apr 4 | Case series | Individual | 4 (out of 18) | HBV(1), HCV(2) , HBV & HCV(1) | OT recipients | 8/10 |
| Guan (33) | China | Dec 2019 to Jan 20 | Cross-sectional | Aggregate | 28 | HBV | COVID-19 patients | 8/9 |
| Hammami (34) | USA | Not reported | Case report | Individual | 1 | HCV | LT recipient | 7/8 |
| Huang (35) | China | Feb 1 to Mar 16 | Case report | Individual | 1 | HBV | LT recipient | 7/8 |
| John Hann (36) | UK | Mar 11 to Apr 12 | Case series | Individual | 1 (out of 3) | HCV | LT recipients | 5/10 |
| Kates (37) | USA | Not reported | Case series | Individual | 1 (out of 4) | HCV | OT recipients | 9/10 |
| Kreivenaite (38) | Lithuania | Mar 16 to Mar 23 | Case report | Individual | 1 | HCV | Cirrhosis | 6/8 |
| Leeb (39) | USA | Mar 18 to Apr 13 | Case series | Aggregate/Individual | 2 (out of 28) | HCV | LT recipients | 10/10 |
| Li (40) | China | Jan 18 to Feb 26 | Case series | Individual | 7 | HBV | HBV patients | 10/10 |
| Liu (41) | China | Jan 23 to Feb 28 | Case report | Individual | 1 | HBV | LT recipient | 5/8 |
| Liu, J.(42) | China | Jan 1 to Apr 12 | Cross sectional | Aggregate | 21 | HBV | HBV patients | 9/9 |
| Loinaz (43) | Spain | Mar 15 to May 5 | Case series | Individual | 9 (out of 19) | HBV(2), HCV(7) | LT recipients | 9/10 |
| Machado (44) | Brazil | March 31 to Apr 12 | Case report | Individual | 1 | HCV | KT recipient | 7/8 |
| Müller (45) | Austria | Mar 11 to Apr 9 | Case report | Individual | 1 | HCV(HIV+) | HCV patient | 6/8 |
| Patrono (46) | Italy | Mar 5 to Apr 10 | Case series | Individual | 3 | HBV(2), HCV(1) | LT recipients | 6/10 |
| Qi (47) | China | Jan 28 to Mar 11 | Case series | Individual | 1 (out of 3) | HBV | Cirrhosis | 7/10 |
| Qin(48) | China | Jan 14 to Mar 9 | Case report | Individual | 1 | HBV | LT recipient | 6/8 |
| Song (49) | China | Dec 2019 to Mar | Case report | Individual | 1 (out of 4) | HBV | LT recipients | 5/10 |
| Waisberg (50) | Brazil | Mar to May | Case series | Individual | 1 (out 5) | HCV | LT recipients | 9/10 |
| Zhang (51) | China | Jan 24 to Feb 29 | Cross-sectional | Aggregate | 23 | HBV | HBV patients | 9/9 |
| Zhao (52) | China | Jan 25 to Jan 30 | Case report | Individual | 1 | HCV(HIV+) | HCV patient | 6/8 |
| Zhong (53) | China | Jan 14 to Mar 12 | Case series | Individual | 1 (out of 2) | HBV | OT recipients | 6/10 |
| Zou (54) | China | Feb 1 to Mar 10 | Cross-sectional | Aggregate | 105 | HBV | HBV patients | 9/9 |
Abbreviations: LT: Liver Transplant, OT: Organ Transplant, KT: Kidney Transplant.
aStudy periods refer to 2020 unless otherwise specified.
bLee, B et al. reported COVID-19 in 38 LT recipients, among whom 16 were HCV-positive and two were HBV-positive, but individual information was reported only for the deceased patients that included seven of total LT recipients and two of HCV-positive patients. For 14 HCV-positive and two HBV-positive patients, there was no individual information and we only knew that they were cured at the end of the follow-up.
6.2. Participant Characteristics
As shown in Table 1, study populations were diverse and included organ transplant patients (15 studies), people living with HBV and/or HCV (nine studies), cirrhosis patients (two studies), and a diverse group of COVID-19 patients with hepatic comorbidities (one study). Across the 27 studies, 232 SARS-CoV-2 infections were found in patients with HBV and 22 in patients with HCV. Overall, the mean (SD) age was 49.9 (9.7) and 62.8 (10.3) among patients with HBV and HCV, respectively. Most patients in both groups were male (61.6% among patients with HBV and 72.8% among patients with HCV).
Among SARS-CoV-2-HBV co-infected patients recruited in cross-sectional studies, no one had a history of liver transplantation or immunosuppressive therapy, and only 3.1% (five out of 163) had cirrhosis. These conditions, however, were more commonly reported in case series and case reports of SARS-CoV-2-HBV co-infected patients (Table 2). Among SARS-CoV-2-HCV co-infected patients recruited in case series/reports, 95.2% (20 out of 21) had cirrhosis, and 90.9% (20 of 22) had a history of liver transplantation and immunosuppressive therapy (Table 3).
| Characteristics (n Reported) | Cross-Sectional; No. (%) | Case Series/Reports; No. (%) |
|---|---|---|
| Age (Mean ± SD) | 49.6 ± 8.7 [n = 87] | 50.8 ± 13.5 [n = 20] |
| Sex | n = 212 | n = 20 |
| Male | 124 (58.5) | 19 (95) |
| Female | 88 (41.5) | 1 (5) |
| Cirrhosis | n = 163 | n = 20 |
| Yes | 5 (3.1) | 13 (65) |
| Liver transplantation | n = 163 | n = 20 |
| Yes | 0 (0) | 11 (55) |
| Immunosuppressive therapy | n = 163 | n = 20 |
| Yes | 0 (0) | 11 (55) |
| Elevated liver biochemistries | n = 150 | n = 13 |
| ALT | 33 (22.0) | 7 (53.8) |
| AST | 39 (26.0) | 4 (30.8) |
| ALP | 3 (2.0) | 1 (7.7) |
| GGT | 20 (13.3) | 4 (30.7) |
| Co-morbidities (in addition to COVID-19/hepatitis) | n = 164 | n = 15 |
| At least one morbidity | 57 (34.7) | 4 (26.7) |
| Hypertension | 33 (20.1) | 2 (13.3) |
| Chronic obstructive pulmonary disease | 3 (1.8) | 2 (13.3) |
| Diabetes | 12 (7.3) | 4 (26.7) |
| Cardiovascular disease | 8 (4.9) | 0 |
| Malignancy | 8 (4.9) | 0 |
| COVID-19 symptoms | n = 168 | n = 20 |
| Fever | 130 (77.4) | 17 (86.0) |
| Cough | 99 (58.9) | 13 (65.5) |
| Dyspnea | 66 (39.3) | 8 (40.0) |
| Headache | 5 (3) | 0 |
| Arthralgia/myalgia | 5 (3) | 3 (15.0) |
| Fatigue | 58 (34.5) | 2 (10.0) |
| Gastrointestinal symptoms | 23 (13.7) | 5 (25.0) |
| Severitya | n = 212 | n = 18 |
| Non-severe | 128 (60.4) | 13 (72.2) |
| Severe | 84 (39.6) | 5 (27.8) |
| Hospitalization | n = 211 | n = 20 |
| Yes | 211 (100) | 20 (100) |
| Intensive care unit admission | n = 71 | n = 18 |
| Yes | 7 (9.8) | 5 (27.8) |
| Death | n = 211 | n = 20 |
| Yes | 10 (4.7) | 3 (15) |
aMild cases reported as non-severe, and severe/critical cases reported as severe.
| Characteristics (n Reported) | Case Series/Reports; No. (%) |
|---|---|
| Age (Mean ± SD) | 62.8 ± 10.3 [n = 22] |
| Sex | n = 22 |
| Male | 16 (72.8) |
| Female | 6 (27.3) |
| Cirrhosis | n = 21 |
| Yes | 20 (95.2) |
| Liver transplantation | n = 22 |
| Yes | 20 (90.9) |
| Immunosuppressive therapy | n = 22 |
| Yes | 20 (90.9) |
| Elevated liver biochemistries | n = 13 |
| ALT | 5 (38.5) |
| AST | 5 (38.5) |
| ALP | 3 (23.1) |
| GGT | 3 of 9 (33.3) |
| Co-morbidities (in addition to COVID-19/hepatitis) | n = 21 |
| At least one morbidity | 16 (76.2) |
| Hypertension | 12 (57.1) |
| Chronic obstructive pulmonary disease | 1 (4.8) |
| Diabetes | 8 (38.1) |
| Cardiovascular disease | 4 (19) |
| Malignancy | 0 |
| COVID-19 symptoms | n = 22 |
| Fever | 17 (77.3) |
| Cough | 14 (63.6) |
| Dyspnea | 9 (40.9) |
| Headache | 1 (4.5) |
| Arthralgia/myalgia | 4 (18.2) |
| Fatigue | 4 (18.2) |
| Gastrointestinal symptoms | 7 (31.8) |
| Severitya | n = 14 |
| Non-Severe | 11 (78.6) |
| Severe | 3 (21.4) |
| Hospitalization | n = 22 |
| Yes | 19 (86.4) |
| Intensive care unit admission | n = 14 |
| Yes | 3 (21.4) |
| Deathb | n = 36 |
| Yes | 3 (8.3) |
aMild cases reported as non-severe, and severe/critical cases reported as severe.
bOne of the cross-sectional studies reported COVID-19 in 38 LT recipients, among whom 16 were HCV-positive, and two were HBV-positive, but individual information was reported only for the deceased patients that included seven of total LT recipients and two of HCV-positive patients. For 14 HCV-positive and two HBV-positive patients, there was no individual information, and we only knew that they were cured at the end of the follow-up. Thus, we included them only for the calculation of the proportion of death.
6.3. COVID-19-Related Symptoms
As shown in Table 2 and Table 3, several comorbidities other than COVID-19 and HBV/HCV were reported among the participants. Fever, cough, dyspnea, fatigue, and gastrointestinal symptoms were the most commonly reported COVID-19 symptoms.
Having elevated ALT (i.e., > 45) was found in 22% (33 out of 150) of patients with SARS-CoV-2-HBV co-infection in cross-sectional studies and 53.8% (seven out of 13) in case series/report studies. In addition, elevated AST (i.e., > 45) was found in 26% (39 out of 150) of patients with SARS-CoV-2-HBV co-infection in cross-sectional studies and 30.8% (four out of 13) in case series/report studies. In patients with SARS-CoV-2-HCV co-infection, 38.5% (five out of 13 patients) had elevated ALT and AST.
All of the patients in the SARS-CoV-2-HBV co-infected group and 86.4% (19 of 22) of cases in the SARS-CoV-2-HCV co-infected group were hospitalized. The ICU admission in the SARS-CoV-2-HBV group was reported in 9.8% (seven of 71) of patients in cross-sectional studies and 27.8% (five of 18) in case series/report studies. The ICU admission was reported in 21.4% of patients in the SARS-CoV-2-HCV group. In the SARS-CoV-2-HBV group, severe COVID-19 symptoms were reported in 39.6% (84 of 212) of patients in cross-sectional studies and 27.8% (five of 18) in case series/report studies. In the SARS-CoV-2-HCV group, severe COVID-19 symptoms were reported in 21.4% (three of 14) of patients.
6.4. Death Proportion Among the Patients
Overall, the death proportions were 4.7% (10 out of 211) and 15% (3 out of 20) among SARS-CoV-2-HBV co-infected patients in cross-sectional and case series/report studies, respectively. The three deceased patients in case series/report studies were 73, 53, and 59-years-old and all were males. They had more than one comorbidity, and two of them were on immunosuppressive therapy due to their history of liver transplantation. Information on age and sex of the deceased patients among SARS-CoV-2-HBV co-infected patients in cross-sectional studies was available for two out of 10 patients. Both were male with 38 and 74 years of age.
The proportion of death among SARS-CoV-2-HCV co-infected patients was 8.3 (three of 36). These three patients were 69, 71, and 79-years-old. Two of them were female, and one was male. They had more than one comorbidity and were on immunosuppressive therapy due to their history of liver transplantation.
6.5. COVID-19 Among Immunosuppressed Patients
Based on the available individual data, the mean (SD) age of immunosuppressed patients was significantly higher than that of patients without immunosuppression (60.8 [11.7] vs. 49.9 [12]; P value = 0.001). About 85% (n = 29) of immunosuppressed patients and 66% (n = 31) of patients without immunosuppression were male (P value = 0.04). Overall, 51.5% (n = 17) of patients with immunosuppression and 30% (n = 12) of patients without immunosuppression had at least one comorbidity other than COVID-19 and hepatitis (P value = 0.07). Gastrointestinal symptoms were more common in immunosuppressed patients (32.2% vs. 7.3%; P value = 0.03). The proportions of severe COVID-19 in patients with and without immunosuppression were 33.3% and 30.4%, respectively (P value = 0.7). About 29% of patients with immunosuppression and 12.5% of patients without immunosuppression were admitted to the ICU (P value = 0.1). Death happened in 17.6% of patients with immunosuppression and 6.4% of patients without immunosuppression (P value = 0.1). See Table 4 for further details.
| Characteristics (n reported) | IS therapy, No. (%) | No IS Therapy, No. (%) | P Value |
|---|---|---|---|
| Age (Mean ± SD) | (n = 31) 60.8 ± 11.7 | (n = 22) 49.9 ± 12 | 0.001 |
| Sex | n = 34 | n = 47 | |
| Male | 29 (85.3) | 31 (66) | 0.04 |
| Female | 5 (14.7) | 16 (34) | |
| Co-morbidities (in addition to COVID-19 and hepatitis) | n = 33 | n = 40 | |
| At least one morbidity | 17 (51.5) | 12 (30) | 0.09 |
| COVID-19 symptoms | n = 34 | n = 47 | |
| Fever | 27 (79.4) | 29 (61.7) | 0.1 |
| Cough | 20 (58.8) | 23 (48.9) | 0.3 |
| Dyspnea | 13 (38.2) | 9 (19.1) | 0.09 |
| Headache | 1 (2.9) | 2 (4.2) | 0.3 |
| Arthralgia/myalgia | 6 (17.6) | 4 (8.5) | 0.2 |
| Fatigue | 6 (17.6) | 9 (19.1) | 0.1 |
| Gastrointestinal symptoms | 11 (32.3) | 3 (7.3) | 0.03 |
| Severity | n = 24 | n = 46 | |
| Non-severe | 16 (66.7) | 32 (69.6) | 0.7 |
| Severe | 8 (33.3) | 14 (30.4) | |
| Hospitalization | n = 34 | n = 47 | 0.07 |
| Yes | 31 (91.2) | 47 (100) | |
| Intensive care unit admission | n = 24 | n = 32 | 0.1 |
| Yes | 7 (29.2) | 4 (12.5) | |
| Death | n = 34 | n = 47 | 0.1 |
| Yes | 6 (17.6) | 3 (6.4) |
6.6. Quality Assessment
As shown in Table 1, quality assessment scores of the studies ranged from five to seven for case reports (out of eight possible points), five to 10 for case series (out of 10 possible points), and eight to nine for cross-sectional studies (out of nine possible points). Further details on the quality assessment tools and respective scores are presented in Supplementary File S3.
7. Discussion
We summarized data from 232 patients with SARS-CoV-2 and HBV co-infection and 22 patients with SARS-CoV-2 and HCV co-infection. While our findings are relatively comparable with evidence from COVID-19 patients without hepatic comorbidities (10, 55), our data pointed to higher morbidity and mortality among COVID-19 patients living with HBV, HCV, or both. The most common COVID-19-related symptoms were fever, cough, dyspnea, fatigue, and gastrointestinal symptoms, which have also been common in COVID-19 patients without these co-infections (13, 56-58). Besides, ALT and AST were elevated in a considerable proportion of the patients, a finding that is consistent with estimates of a previous systematic review of earlier studies on liver manifestations among COVID-19 patients, where the pooled incidence of elevated liver chemistries among COVID‐19 patients was reported as 23.1% (13).
Many studies have reported elevated liver biochemistries to be common among COVID‐19 patients with and without hepatic comorbidities (13, 59, 60); however, these findings should be interpreted with caution. First, it is unclear whether the liver damage observed among COVID-19 patients with HBV or HCV comorbidities is mainly due to the adverse impact of SARS-CoV-2 on hepatic cells or patients’ pre-existing viral hepatitis. Second, corticosteroids that are commonly used as a treatment strategy in COVID-19 patients have been shown to increase the risk of hepatitis flare in HBV patients and might impact liver enzyme profiles among these patients (61-63). On the other hand, Chen et al. reported no difference in the level of liver function parameters between COVID-19-infected patients and COVID-19-HBV co-infected patients (30). Kulkarni et al.'s meta-analysis of COVID-19-related liver manifestations reported patients with pre-existing chronic liver disease to have lower odds of developing severe COVID-19 (13). All of these rather equivocal findings highlight the importance of conducting well-controlled studies to fully understand the pathogenesis of SARS-CoV-2 among HBV and HCV patients and determine the main underlying causes of elevated liver enzymes among these multimorbid patients to help improve clinical decision-making.
All of the patients in the SARS-CoV-2-HBV group and 86.4% of cases in the SARS-CoV-2-HCV group were hospitalized. While this finding might be interpreted as a higher risk of hospitalization among these multimorbid populations, it could also be reflective of the fact that the existing evidence is mainly based on, and indeed, skewed towards hospitalized severe COVID-19 patients with hepatic comorbidities. This was further evident in our finding that ICU admission in the SARS-COV-2-HBV co-infected group was estimated at 9.8% and 27.8% in cross-sectional and case series/report studies, respectively. Additionally, ICU admission in the SARS-COV-2-HCV co-infected group was estimated at 21.4%.
The proportions of death were 4.7% and 15% among reported cases of SARS-CoV-2-HBV co-infection in cross-sectional and case series/report studies, and 8.3% of reported cases in SARS-CoV-2-HCV co-infection. Given the high prevalence of multimorbidity among HBV and HCV patients and the fact that most patients included in this review were severe cases of hospitalized COVID-19 with a history of organ transplant, this finding should also be interpreted with caution when compared to the overall risk of mortality among COVID-19 patients, estimated to be around 2% (10). Nonetheless, these findings are informative and suggest that among those who died, multimorbidity, older age, and male sex were common in comparison with all included patients.
Our findings of the impact of immunosuppression on COVID-19 severity are interesting, given the equivocal findings of different studies on the susceptibility of immunosuppressed patients to severe SARS-CoV-2 infection. While some assume a higher risk for the immunosuppressed population due to their systemic immunocompromised status (21, 64), others suggest that immunosuppression may protect against the hyperactivated immune response (65, 66). We found that the proportions of severe COVID-19 cases, admission to the ICU, and death were higher among patients with immunosuppression than in patients without immunosuppression; however, these differences were not statistically significant. These findings are not conclusive and should be interpreted with an eye for the limited sample size in the studies, the observational nature of the studies, the non-matched nature of the comparisons, and the fact that these differences could be due to the immunosuppressed group being more likely to be older, male, and have organ transplants, advanced liver disease, and other extrahepatic comorbidities.
We acknowledge the limitations of our study. First, while most COVID-19 patients are asymptomatic, the available evidence that informed this review included mostly hospitalized patients and was skewed towards more severe patients often with a history of organ transplant and advanced liver disease; therefore, our findings cannot be generalizable to all patients living with COVID-19 and HBV or HCV comorbidities. Second, due to the descriptive design of the included studies and lack of a comparison group, we could not identify factors associated with SARS-CoV-2-HBV or HCV co-infections. Third, most of the included studies were case series and had a small sample size. Therefore, the accurate prevalence of COVID-19 and its manifestations among HBV or HCV subpopulations remains unknown without a population-based survey of patients with these comorbidities. Lastly, given the growing nature of the pandemic and the overwhelmed healthcare systems worldwide, viral hepatitis manifestations may be under-recorded or overlooked during clinical visits, leading to the underestimation of the scope of hepatic manifestations among COVID-19 patients with HBV and HCV comorbidities.
7.1. Conclusions
Despite the limitations of the existing evidence, our data suggest that liver enzyme abnormalities and acute hepatic injuries may be common among COVID-19 patients with HBV and HCV comorbidities. Therefore, these paraclinical profiles should be monitored and examined during clinical visits. While understanding the pathogenesis of SARS-CoV-2 requires further investigations, a careful assessment of hepatic manifestations upon admission could help reduce the multimorbidity among HBV or HCV patients and lead to more favorable health outcomes among COVID-19 patients.