1. Background
Hepatitis C virus (HCV) infection remains a global public health threat. Its chronic stage is commonly associated with potentially fatal complications, ranging from cirrhosis, liver failure to hepatocellular carcinoma (HCC) (1). Approximately 71 million individuals are currently living with HCV infection, and nearly 400,000 individuals died from its complications in 2016 alone (2). In Malaysia, it is estimated that 1.9% of its population are infected with HCV (3), mainly of genotype 3 (61.9%) and 1 (35.9%) (4). Blood and blood product transfusion, intravenous drug use, incarceration, tattooing, body piercing and having multiple sexual partners are among the common risk factors of hepatitis C in the country (5).
The interferon-based regimen was once the backbone of hepatitis C treatment, despite its relatively low post-treatment sustained virologic response (SVR) rate (40 - 50%) and unfavorable safety profile (6). Over the last decade, hepatitis C treatment reached an important milestone with the advent of all-oral direct-acting antivirals (DAAs). NS3/4A protease inhibitors, NS5A inhibitors and NS5B polymerase inhibitors, the three major classes of DAAs, have commonly been used in combination and promise a better treatment outcome as compared with the conventional treatment (7). They also demonstrate good tolerability in general, even in patients who are co-infected with human immunodeficiency virus (HIV) (8).
However, as the primary source of medical care for the Malaysian population, public hospitals have been under tremendous budgetary pressure to provide hepatitis C patients with no-cost treatment. Despite their improved effectiveness and safety profile, the use of DAAs was once limited in Malaysia, mainly attributable to their prohibitively high costs. A major change only took place in late 2017, the year in which Malaysia became the first country invoking the agreement on trade-related aspects of intellectual rights and issuing a compulsory license to a generic version of sofosbuvir (9). Such a decision was made after rounds of unsuccessful price negotiation with the patent-holder of sofosbuvir, which forms part of many recommended DAA regimens. Afterward, the generic version of sofosbuvir has widely been used together with daclatasvir, which is also available in the generic form in Malaysia, as the standard treatment for hepatitis C in public hospitals across the country (10).
The sofosbuvir-daclatasvir combination is one of the WHO-recommended pangenotypic DAA regimens for hepatitis C treatment (11). It was consistently shown to produce an SVR rate above 90% in non-cirrhotic and 80% in cirrhotic patients worldwide (12-32). It is noteworthy that some of these studies generated their findings from either a clinical trial or a structured treatment accessibility program (12-15). A few of them were undertaken in settings in which the sofosbuvir-daclatasvir combination only served as an option among many others (16-23), while the rest presented the data limited to specific populations, HCV genotypes and liver cirrhotic status (24-32).
Nevertheless, in a context like Malaysia, the effects of the “one-size-fits-all strategy” with the use of a fixed DAA regimen on all public health settings are still unclear. Although patented, high-priced alternatives are not widely available in Malaysia, the concern is raised about the performance of the generic forms of DAAs used on a large scale in hepatitis C treatment.
2. Objectives
Therefore, this study was designed to comprehensively evaluate the outcomes of such a government-led initiative in multiple aspects, including the number and characteristics of patients treated, the extent of evidence-based drug use, the treatment completion status, individual responses to treatment, common side effects of treatment, and its economic implications.
3. Methods
3.1. Study Design
This was a nationwide retrospective cohort study complemented by a drug cost analysis. The clinical data were obtained from 16 public hospitals selected by the National Gastroenterology and Hepatology Services (NGHS) Committee of the Ministry of Health (MOH). Each of the 14 states of Malaysia was represented by at least one hospital, which had been actively offering no-cost DAA-based treatment to hepatitis C patients since the second quarter of 2018. They were either secondary or tertiary hospitals, staffed with at least one gastroenterologist or physician each. The drug cost data was collected from the Pharmaceutical Services Program, which acquired DAAs for all the 16 hospitals under central contracts. The study was granted with the registration number (NMRR-20-481-54147) by the National Medical Research Register and approved by the Medical Research Ethics Committee of the MOH.
3.2. Clinical Data Collection
The data were collected from April to June 2020. A gastroenterologist or physician from each hospital was assigned by the NGHS Committee to retrieve and verify the data from medical records of all the hepatitis C patients treated with sofosbuvir and daclatasvir from 1 April 2018 and 31 March 2020. Patients were eligible if they were (1) above 18 years of age; (2) had their diagnosis confirmed by either the HCV core antigen or ribonucleic acid (RNA) test; and (3) received treatment with an 8- or 12-week course of sofosbuvir and daclatasvir, with or without ribavirin.
The information gathered for each patient ranged from (1) their baseline characteristics, including age, gender, ethnicity, and their history of exposure to risk factors of HCV infection; (2) their comorbidities, including HIV infection, hepatitis B virus infection, and chronic kidney disease (defined as an estimated glomerular filtration rate < 60 mL/min/1.73m2 lasting for > 3 months); (3) their HCV infection history, including the HCV genotype, and their experience with interferon-based treatment; (4) the presence and the Child-Turcotte-Pugh (CTP) classification of liver cirrhosis; (5) their treatment history, including the date of treatment initiation, dose, the treatment duration, the use of ribavirin, and the treatment completion status (self-reported by patients during clinic visits, cross-checked with prescription-refill records or patient diary); (6) the treatment outcomes, including the achievement of an SVR (defined as an HCV RNA level < 15 IU/mL 12 weeks after the treatment completion), and the presence of common side effects (as listed in the clinical practice guidelines of the MOH and studied side effects, including headache, fatigue, nausea, diarrhea, and anemia) (33).
3.3. Clinical Data Assessment
The number of patients receiving treatment over the two-year period was tallied, and the distribution of their demographic and clinical characteristics was studied. The assessment of the extent of evidence-based drug use was performed according to the clinical practice guidelines of the MOH (33). It was recommended by the MOH that sofosbuvir and daclatasvir be used for 12 or 24 weeks at a fixed, once-daily dose of 400 mg and 60 mg, respectively. Dose adjustment was only required for daclatasvir in HCV/ HIV co-infected patients treated with antivirals potentially causing drug-drug interactions (30 mg for CYP3A4 inhibitors and 90 mg for CYP3A4 inducers). The use of ribavirin as an adjunct to the two-drug combination could be considered for (1) genotype-3 HCV infection complicated with liver cirrhosis; and (2) genotype-1a HCV infection in interferon-experienced patients. The recommended daily doses for ribavirin were, respectively, 1,200 mg and 1,000 mg for a bodyweight above and below 75 kg.
The treatment completion status up until 31st of March 2020 was summarized as (1) complete treatment with the SVR test result; (2) complete treatment pending the SVR test or test result; (3) ongoing treatment (within the prescribed treatment duration); and (4) incomplete treatment. The reasons for incomplete treatment were further classified into (1) loss to follow-up during the treatment period; (2) discontinuation due to disease advancement (HCC, liver failure, hepatitis-related complications, or exacerbation of other chronic conditions); (3) discontinuation due to intolerable side effects; and (4) death during the treatment period.
The SVR rate was used as the measure for individual responses to treatment. It represented the proportion of patients achieving an SVR to those who had known treatment outcomes. In this regard, the known treatment outcomes included achieving an SVR; failure to achieve an SVR; and premature treatment discontinuation due to intolerable side effects, disease advancement or death. Those whose SVR test results were unavailable or treatment was still ongoing as at 31st of March 2020, along with those who were lost to follow-up during the treatment period, were excluded from the treatment outcome analysis. In addition to the overall SVR rate and the factors associated with the treatment failure, the SVR rates for all the patient subgroups were also presented. In addition, the rates of common side effects of the treatment, along with the rates of side effects causing the treatment discontinuation, were determined.
3.4. Statistical Analysis
The clinical data were managed and analyzed by using the SPSS for Windows version 21.0 (IBM, New York). The findings were mainly summarized as frequencies and percentages. The SVR rates were presented as percentages and 95% confidence intervals (CIs). The factors associated with the failure to achieve an SVR were further explored by using the simple and backward stepwise multiple logistic regression analyses, with the findings expressed as odds ratios (ORs) and 95% CIs. The final model was tested for the interactions and multicollinearity between variables, as well as for its fitness using the Hosmer-Lemeshow goodness-of-fit test, the overall correctly classified percentages and the area under the receiver operating characteristic (ROC) curve. The significant level of all the statistical tests was fixed at 0.05.
3.5. Drug Cost Analysis
The mean unit costs (per tablet) of three drugs (sofosbuvir, daclatasvir and ribavirin) were calculated by dividing their total acquisition costs by the number of tablets acquired within the two-year period. The expenditure on each drug was estimated by multiplying their mean unit costs (per tablet) with the number of patients and treatment duration. In addition to the total drug expenditure, the average cost of a 12-week course of treatment of sofosbuvir and daclatasvir (with and without ribavirin) was computed. The findings were presented in US$, with the conversion performed based on the mean exchange rate in 2019 (US$ 1= MYR 4.14).
4. Results
4.1. Number and Characteristics of the Patients
A total of 1,797 hepatitis patients were treated with sofosbuvir and daclatasvir over a two-year period. The number of patients receiving the treatment ranged from 11 to 271 across the hospitals. They were mainly male (74.1%), below 50 years of age (51.4%) and of Malay ethnicity (60.2%). Approximately 4 in every 10 of them had a history of intravenous drug use, and nearly 10% of them were co-infected with HIV. The most common HCV genotype was 3 (46.9%), followed by 1a (20.0%), 1b (8.7%) and 2 (2.4%). However, the HCV genotype in approximately one-fifth of them was untested. More than one-third of them had liver cirrhosis, mainly of CTP class A (31.4%). Only 9.1% of them were interferon-experienced (Table 1).
| Characteristics | No. (%) |
|---|---|
| Gender | |
| Male | 1,331 (74.1) |
| Female | 466 (25.9) |
| Age (y) | |
| < 50 | 923 (51.4) |
| ≥ 50 | 874 (48.6) |
| Ethnicity | |
| Malay | 1,081 (60.2) |
| Chinese | 457 (25.4) |
| Indian | 94 (5.2) |
| Others | 165 (9.2) |
| History of exposure to HCV risk factors a | |
| Intravenous drug use | 731 (40.7) |
| Invasive medical procedures/blood transfusion | 327 (18.2) |
| Sexual contact | 245 (13.6) |
| Tattooing | 85 (4.7) |
| Hemodialysis | 9 (0.05) |
| Body piercing | 13 (0.07) |
| Presence of HBV infection | 43 (2.4) |
| Presence of HIV infection | 165 (9.2) |
| Presence of CKD | 26 (1.4) |
| History of interferon-based treatment | 163 (9.1%) |
| Genotype of HCV | |
| 1a | 359 (20.0) |
| 1b | 157 (8.7) |
| 2 | 43 (2.4) |
| 3 | 842 (46.9) |
| 4 | 4 (0.2) |
| 6 | 4 (0.4) |
| Untested | 388 (21.6) |
| Presence of liver cirrhosis | |
| No | 1,149 (64.0) |
| CTP class A (score 5 - 6) | 565 (31.4) |
| CTP class B (score 7 - 9) | 79 (4.4) |
| CTP class C (score 10 - 15) | 4 (0.2) |
| Treatment duration of SOF/DAC | |
| 12 weeks | 1,365 (76.0) |
| 24 weeks | 432 (24.0) b |
| Use of ribavirin together with SOF/DAC | 410 (22.8) c |
Abbreviations: CKD, chronic kidney disease; CTP, Child-Turcotte-Pugh; DAC, daclatasvir; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; SOF, sofosbuvir.
a A patient could be linked to more than one risk factor.
b Mainly used for genotype-3 HCV infection complicated with liver cirrhosis (n = 352; 81.5%).
c Mainly used for genotype-3 HCV infection complicated with liver cirrhosis (n = 314; 76.6%) and genotype-1a HCV infection in interferon-experienced patients (n = 32; 7.8%).
4.2. Extent of Evidence-based Drug Use
It was found that the drugs were generally used in line with the recommendations of the MOH. All of the patients were found to have received both DAAs at the right dose. This included 122 (6.8%) HCV/HIV co-infected patients for whom dose adjustment was required; 112 received daclatasvir at 90 mg and 10 at 30 mg daily. The treatment was extended to 24 weeks in 24.0% of the patients, more than 80% of whom had genotype-3 HCV infection complicated with liver cirrhosis. Ribavirin was used in slightly more than one-fifth of the patients, particularly those who had genotype-3 HCV infection complicated with liver cirrhosis (76.7%); and those who had genotype-1a HCV infection and were interferon-experienced (7.8%) (Table 1).
4.3. Status of Treatment Completion
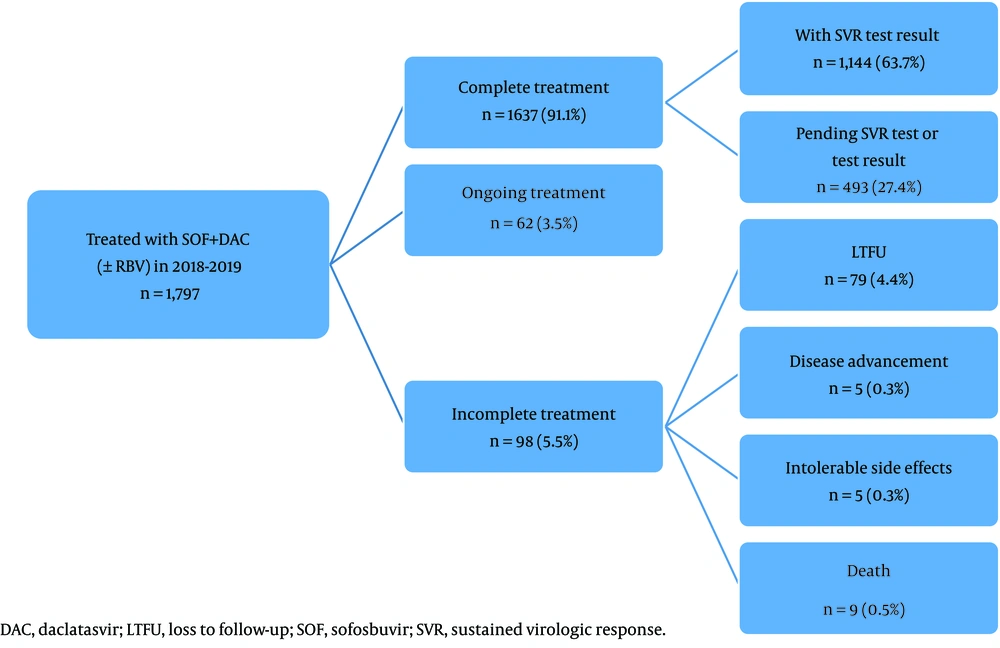
Up until the end of March 2020, slightly over 90% of the patients had completed their treatment, most of whom had undergone the SVR test and the results were available (63.7%). Incomplete treatment was reported for 98 (5.5%) patients, primarily resulting from the loss to follow-up (4.4%). The premature treatment discontinuation in the rest was mainly caused by death (0.5%), followed by intolerable side effects (0.3%) and disease advancement (0.3%) (Figure 1).
4.4. Individual Responses to Treatment
Of the 1,163 patients with known treatment outcomes, 1,110 patients achieved an SVR. This yielded an overall SVR rate of 95.4% (95% CI: 94.2, 96.7%). The SVR achievement did not vary across HCV genotypes and the liver cirrhosis status of patients. The treatment duration, the use of ribavirin, and the previous exposure to interferon were also shown to have no effect on the SVR achievement (Table 2). However, treatment failure more likely occurred in the patients who were above 50 years of age (adjusted OR: 2.13; 95% CI: 1.16, 3.92) or had a history of tattooing (adjusted OR: 4.77; 95% CI: 1.85, 12.32) (Table 3).
| Factors | Number of Patients Achieving SVR | SVR Rate (95% CI) | Crude OR (95% CI) | P-Value |
|---|---|---|---|---|
| Overall | 1,110/1,163 | 95.4 (94.2, 96.7) | - | - |
| Gender | ||||
| Male | 795/838 | 94.9 (93.4, 96.4) | 1.70 (0.85, 3.43) | 0.136 |
| Female | 315/325 | 96.9 (95.0, 98.8) | 1 | - |
| Age (y) | ||||
| < 50 | 536/555 | 96.6 (95.1, 98.1) | 1 | - |
| ≥ 50 | 574/608 | 94.4 (92.6, 96.2) | 1.67 (0.94, 2.97) | 0.079 |
| Ethnicity | ||||
| Malay | 676/702 | 96.3 (94.9, 97.7) | 1 | - |
| Chinese | 289/309 | 93.5 (90.8, 96.3) | 1.80 (0.99,3.28) | 0.055 |
| Indian | 54/58 | 93.1 (86.4, 99.8) | 1.93 (0.65, 5.72) | 0.238 |
| Others | 91/94 | 96.8 (93.2, 100.0) | 0.86 (0.25, 2.89) | 0.804 |
| Intravenous drug use | ||||
| No | 689/723 | 95.3 (93.8, 96.8) | 1.09 (0.62, 1.94) | 0.761 |
| Yes | 421/440 | 95.7 (93.8, 97.6) | 1 | - |
| Invasive medical procedures/blood transfusion | ||||
| No | 884/931 | 95.0 (93.5, 96.4) | 2.00 (0.85, 4.74) | 0.114 |
| Yes | 226/232 | 97.4 (95.4, 99.5) | 1 | - |
| Sexual contact | ||||
| No | 969/1,015 | 95.5 (94.2, 96.7) | 1 | - |
| Yes | 141/148 | 95.3 (91.8, 98.7) | 1.05 (0.46, 2.36) | 0.914 |
| Tattooing | ||||
| No | 1,072/1,119 | 95.8 (94.6, 97.0) | 1 | - |
| Yes | 38/44 | 86.4 (75.8, 96.9) | 3.60 (1.45, 8.94) | 0.006 |
| Hemodialysis | ||||
| No | 1,102/1,155 | 95.4 (94.2, 96.7) | - | - |
| Yes | 8/8 | 100.0 | - | - |
| Body piercing | ||||
| No | 1,106/1,159 | 95.4 (94.2, 96.6) | - | - |
| Yes | 4/4 | 100.0 | - | - |
| Presence of HBV infection | ||||
| No | 1,084/1,135 | 95.5 (94.3, 96.7) | 1 | - |
| Yes | 26/28 | 92.9 (82.7, 100.0) | 1.64 (0.38, 7.08) | 0.511 |
| Presence of HIV infection | ||||
| No | 1,011/1,056 | 95.7 (94.5, 97.0) | 1 | - |
| Yes | 99/107 | 92.5 (87.5, 97.6) | 1.82 (0.83, 3.96) | 0.134 |
| Presence of CKD | ||||
| No | 1,094/1145 | 95.5 (94.3, 96.7) | 1 | - |
| Yes | 16/18 | 88.9 (72.8, 100.0) | 2.68 (0.60, 11.98) | 0.196 |
| History of interferon-based treatment | ||||
| No | 987/1,034 | 95.5 (94.3, 96.7) | 1 | - |
| Yes | 123/129 | 95.3 (91.7, 99.0) | 1.02 (0.43, 2.45) | 0.957 |
| Genotype of HCV | ||||
| 1a | 267/279 | 95.7 (93.3, 98.1) | 1 | - |
| 1b | 118/122 | 96.7 (93.5, 99.9) | 0.75 (0.24, 2.39) | 0.631 |
| 2 | 21/22 | 95.5 (86.0, 100.0) | 1.06 (0.13, 8.55) | 0.957 |
| 3 | 530/561 | 94.5 (92.6, 96.4) | 1.30 (0.66, 2.58) | 0.449 |
| Others a | 174/179 | 97.2 (94.8, 99.6) | 0.64 (0.22, 1.85) | 0.408 |
| Presence of liver cirrhosis | ||||
| No | 725/746 | 97.2 (96.0, 98.4) | 1.55 (0.89, 2.70) | 0.120 |
| Yes | 388/398 | 97.5 (95.9, 99.0) | 1 | - |
| Treatment duration | ||||
| 12 weeks | 876/900 | 97.3 (96.3, 98.4) | 1 | - |
| 24 weeks | 237/244 | 97.1 (95.0, 99.2) | 1.46 (0.79, 2.70) | 0.226 |
| Ribavirin use | ||||
| No | 862/885 | 97.4 (96.4, 98.5) | 1 | - |
| Yes | 251/259 | 96.9 (94.8, 99.0) | 1.62 (0.90, 2.94) | 0.109 |
Abbreviations: CKD, chronic kidney disease; CI, confidence interval; CTP, Child-Turcotte-Pugh; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; OR, odds ratio; SVR, sustained virologic response.
a SVR rates were 100% (3/3) for genotype 4, 100% (2/2) for genotype 6, and 97.1% (169/174; 95%CI: 94.6%, 99.6%) for untested cases.
| Variables | Adjusted OR (95% CI) a | P-Value |
|---|---|---|
| Age (y) | ||
| < 50 | 1 | - |
| ≥ 50 | 2.13 (1.16, 3.92) | 0.015 |
| Tattooing | ||
| No | 1 | - |
| Yes | 4.77 (1.85, 12.32) | 0.001 |
Abbreviations: CI, confidence interval; HCV, hepatitis C virus; HIV, human immunodeficiency virus; OR, odds ratio.
a Backward stepwise multiple logistic regression analysis was performed. Multicollinearity and interaction between variables were checked and not found. The Hosmer-Lemeshow goodness-of-fit test (P = 0.958), the overall correctly classified percentage (95.4%) and the area under the receiver operating characteristic curve (62.3%) were used to confirm the model fitness.
4.5. Common Side Effects
Only 0.9% of the patients experienced the common side effects of the treatment. The most frequently reported side effects were anemia (0.6%), followed by fatigue (0.2%) and headache (0.1%). Premature treatment discontinuation due to intolerable side effects only took place in three patients with anemia (0.2%) and two patients with fatigue (0.1%) (Table 4).
| Side Effects | Number of Patients | Number of Patients Whose Treatment Was Discontinued |
|---|---|---|
| Anemia | 11 (0.6) | 3 (0.2) |
| Fatigue | 4 (0.2) | 2 (0.1) |
| Headache | 2 (0.1) | 0 (0.0) |
a Values are expressed as No. (%).
4.6. Drug Expenditure
The drug expenditure added up to US$678,258.20 (US$377.44 per patient on average). Daclatasvir composed the largest portion of the expenditure (US$308,523.88; 45.5%), followed by sofosbuvir (US$225,225.91; 33.2%) and ribavirin (US$114,508.41; 21.3%). The addition of ribavirin was found to increase the mean cost of a 12-week treatment course of sofosbuvir and daclatasvir by 45.2% (US$429.13 versus US$235.16).
5. Discussion
While Malaysia positioned HCV infection as a public health threat and applied compulsory licensing as a policy tool to enhance the accessibility of hepatitis C treatment, this study signifies that the MOH’s decision to use a fixed regimen is comprised of two generic DAAs as a “one-size-fits-all strategy” for the entire country that is arguably reasonable and timely. Even though the pairing of sofosbuvir and daclatasvir would no longer be recommended as the first-line option for hepatitis C treatment (34), it was still shown to be highly efficacious and yet safe in the real world. From the economic standpoint, this study also points to the sustainability of such a strategy, showing that the mean cost of a 12-week treatment course of the two drugs (US$235.16) had been kept below the cost expected by the MOH (US$300) since their first use.
Most likely attributable to the guidance provided through the clinical practice guidelines (33) and continuous learning via various platforms, it is found that the 16 selected hospitals in this study all managed to practice evidence-based drug use in general. The combined use of sofosbuvir and daclatasvir, with or without ribavirin, recorded an overall SVR rate above 95%. This is consistent with the findings of most previous studies, including some undertaken in a controlled environment (13, 16-18, 20, 23, 25, 27, 29). Aside from producing similar outcomes across HCV genotypes and the liver cirrhosis status of patients, the treatment also yielded a comparable SVR rate for the harder-to-treat genotype-3 HCV infection. This strengthens the confidence of the MOH to treat more advanced hepatitis C cases and extend the existing practice to include more health institutions going forward. More importantly, this study could also help dispel the doubts about the efficacy of generic DAAs among both health professionals and the public.
Furthermore, the side effects of the treatment were found to be rare, and if present, were tolerable most of time. Anemia, which was most likely induced by ribavirin (33, 35), emerged as the most frequently reported side effect. Apart from its safety profile, this study raises the concern about the increase in cost by nearly 50% when ribavirin was added to a 12-week treatment course of sofosbuvir and daclatasvir. Although the three-drug combination has primarily been used for genotype-3 HCV infection complicated with liver cirrhosis in Malaysia, this study also suggests that the presence of ribavirin would not significantly improve the treatment outcome. Nevertheless, as a more detailed analysis is not feasible in this study due to a relatively small number of patients in this subgroup, more studies are required to corroborate the findings. Given that the WHO also does not specifically recommend the addition of ribavirin to the sofosbuvir-daclatasvir regimen in any conditions (11), the MOH is looking at the possibility of revising the clinical practice guidelines and further simplifying the treatment for hepatitis C.
It is also encouraging to note that the total number of patients receiving DAA-based treatment at the 16 selected hospitals alone reached 1,797 over the span of two years, nearly four times more than the patients recorded by the MOH to have received treatment with interferon and ribavirin across the country between January 2016 and March 2018. However, there were no significant changes in the expenditure on viral hepatitis detected over the last few years, this implies that the expansion of treatment coverage is achievable by using the existing strategy without elevating the budgetary pressure. The substantial growth of the number of patients treated could also be ascribed to the collaborations between the MOH and civil society organizations, in particular the Drugs for Neglected Diseases initiative (DNDi), the Foundation for Innovative New Diagnostics (FIND) and the Malaysian AIDS Council (MAC), in upscaling the community-based screening for hepatitis C and subsequently linking the patients to hospitals for pharmacological treatment.
As much as the MOH is motivated by the positive findings of this study, it is worth highlighting that not all hepatitis C patients have access to hospital care. Aiming at the global goal to diagnose at least 90% of the HCV-infected individuals and initiate treatment in at least 80% of those who are eligible (36), Malaysia is currently pushing for decentralized hepatitis C management (10). Although public healthcare (PHC) centers in Malaysia generally have limitations with respect to their laboratory facilities and budgets, this study also suggests that ascertaining the genotype of HCV, particularly in non-cirrhotic patients, did not necessarily result in a better treatment outcome. The decision to omit the HCV genotyping at the stage of treatment initiation was arguably acceptable, given that the test results were only essential to guide the use of ribavirin in cases complicated with liver cirrhosis (33). Adopting such a practice in PHC centers can potentially streamline the treatment algorithms and avert unnecessary delay in treatment.
Although most patients were found to have completed their treatment, loss to follow-up was still reported for approximately 5% of them. In addition to expanding the treatment coverage in hepatitis C patients, the MOH, therefore, seek to reduce preventable treatment default. Additionally, this study shows that approximately 4 in every 10 hepatitis C patients treated with DAAs in Malaysia were PWID, who are also known to commonly have multiple encounters with police and the criminal justice system (37). Thus, it is timely for the MOH to forge a partnership with the Ministry of Home Affairs (MHA) in order to ensure the continuity of treatment among the PWID who are imprisoned or followed up at drug rehabilitation centers.
This study also relates the treatment failure in hepatitis C patients to their age and history of tattooing. Regardless of the conflicting conclusions drawn by the previous studies about the link between age and SVR achievement, it is a widely held view that a prolonged duration of HCV infection is likely to increase the risk of hepatitis-related complications and compromise the effectiveness of treatment (38, 39). As tattooing remains a cultural practice in certain areas and one of the key risk factors of hepatitis C in Malaysia (5), the suboptimal responses to treatment in tattooed patients were likely to be associated with their continuous exposure to unsafe practice during the treatment period. Nonetheless, further investigations into the reasons behind the aforementioned relationships are warranted.
The major limitation of this study lies in the short observation period, and the disease progression of the patients following the SVR achievement, along with its economic implications, is unknown. As mounting evidence confirms the long-term effects of DAAs in reducing mortality and the risk of HCC (40), a comprehensive economic evaluation beyond drug costs is required to capture the actual impact of such a government-led initiative. Although Malaysia is moving toward a massive scale-up of hepatitis C treatment through decentralization and outreach programs, this study does not provide insight into how the existing strategy could work as well in other settings as it did in public hospitals. Such uncertainty leaves an important direction for future research.
5.1. Conclusion
This study reveals that in the following two years after generic versions of sofosbuvir and daclatasvir were introduced by the MOH, this two-drug combination had been used to treat nearly 1,800 hepatitis C patients in 16 public hospitals across Malaysia. More than 90% of the patients successfully completed the treatment course. While the drugs were generally used in accordance with the recommendations of the MOH, the treatment resulted in an SVR rate above 95%. Side effects of the treatment were rare and tolerable on most occasions. A standard 12-week treatment course of the two drugs was found to be accessible at an average cost below US$300 throughout the two-year period. Overall, the treatment exhibited a high degree of effectiveness, safety and affordability, pointing to the timeliness of the “one-size-fits-all” strategy to combat hepatitis C in the country.