1. Introduction
Epithelioid hemangioendothelioma (EHE) is a rare neoplasm of vascular origin arising in soft-tissue and different visceral organs, among which the liver is known to be the most commonly involved (1). Hepatic epithelioid hemangioendothelioma (HEHE) is a malignant tumor with an unpredictable clinical course (2). It usually portrays an indolent behavior with a better prognosis than other malignant liver tumors, even when presenting as a diffuse multifocal liver tumor or having simultaneous metastases (3, 4). Yet, this is not always the case since HEHE can be a fatal disease with rapid progression and poor survival (5). There are no reliable clinical and histopathologic factors to predict tumor behavior. In this study, we present three patients with primary EHE of the liver treated by orthotropic transplantation at our center between January 2001 and November 2019.
2. Case Presentation
2.1. Case 1
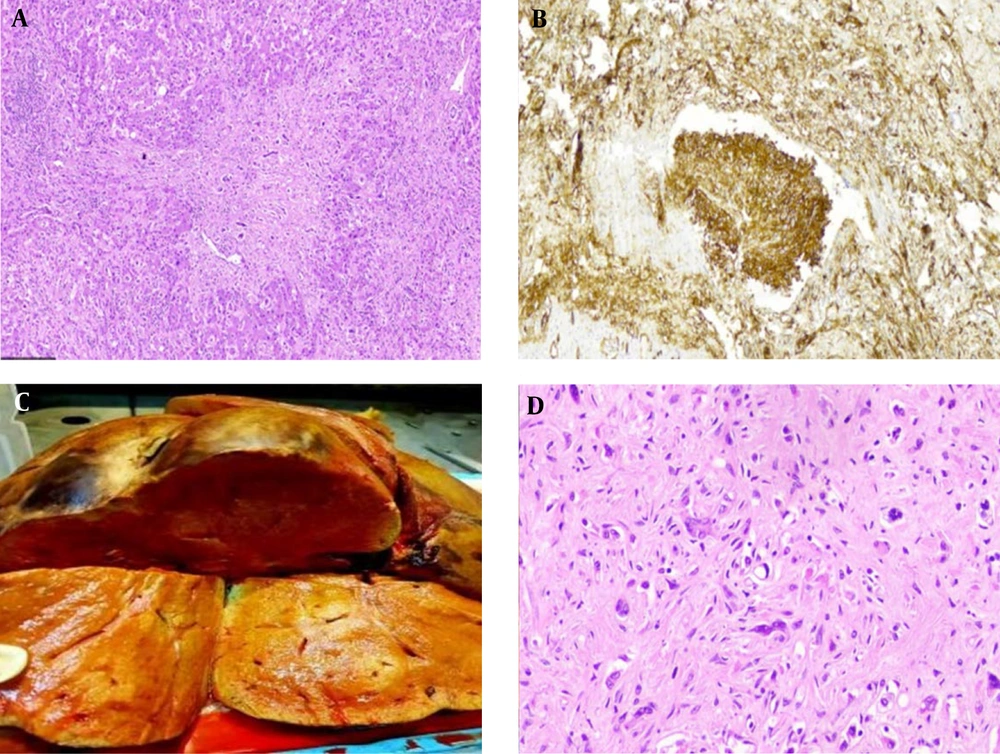
A 49-year-old man underwent an emergency abdominal laparotomy for a perforated peptic ulcer two years before referring to our center in September 2017. During the surgical exploration, it was noticed that the liver was enlarged with a nodular surface. Due to high suspicion, post-operative imaging evaluation was done, which revealed multiple enhancing liver masses throughout the liver parenchyma, predominantly in the left lobe, with few located in the subcapsular area causing surface irregularity. Pathologic study of the performed liver biopsy diagnosed this case as primary liver EHE. The patient was observed for two years until he was admitted to our center with weight loss, abdominal pain, and Budd Chiari syndrome. The preliminary lab workup showed normal levels of liver enzymes and tumor markers. One month later, he received an orthotopic liver transplant. Gross examination of the explanted liver showed multiple tumor masses, ranging from 1 cm to 8 cm in size. Microscopic examination of the explanted liver confirmed the initial diagnosis and showed a multinodular ill-defined infiltrative growth of tumor cells, embedded in a fibrotic myxoid stroma (Figure 1A). The neoplastic cells grew within the sinusoids resulting in hepatocyte atrophy and destruction of the liver cell plates. Intraluminal vascular tufts and complete venular occlusions were seen caused by infiltrating fibrous plugs enclosing epithelioid and spindle cells (Figure 1B). The tumor extended into the wall of large blood channels and also invaded the hilar components and margins. Three hilar lymph nodes showed metastatic involvement. Upon follow-up to this date, the patient is alive and has been free from recurrences or distant metastasis.
(A) A densely fibromyxoid tumor stroma with irregular borders infiltrates the adjacent liver parenchyma (H&E, x40). (B)Immunohistochemical staining with CD34 highlights the tumor intravascular tuft proliferation (IHC, x100). (C) Liver sections show multiple ill-defined cream-colored nodules with a focus of infiltration and retraction of the liver capsule. (D) A sclerotic matrix enclosing epithelioid and stellate dendritic cells. At the center of the field, an epithelioid tumor cell with an intracytoplasmic vacuole is seen, giving the cell a signet ring-like appearance (H&E, x400).
2.2. Case 2
A 55-year-old female came to our hospital in October 2018 with a previous diagnosis of primary HEHE at another center. She had referred to a different medical center due to abdominal pain three months before referral to our center. A liver lobe mass was discovered on abdominal sonography. Upon admission to our center, an abdominal CT scan was done which showed multiple hypodense enhancing liver lesions located in the right and left lobes. Three months later, she was treated by orthotopic liver transplantation.
On gross examination of the explanted liver, an area of capsule retraction was seen on the outer surface inspection. The cut surfaces revealed multiple poorly circumscribed cream-colored masses in both liver lobes (Figure 1C). The largest one was located in the right lobe close to the gallbladder measuring 6 × 6 × 3 cm. It invaded through the Glisson’s capsule into the attached excised diaphragm. Histologically, we observed a sclerotic stroma disrupting the liver parenchyma. On closer look, epithelioid to spindle-like tumor cells were seen seated in the sclerotic hyalinized stroma. The epithelioid cells had round to oval nuclei with slight pleomorphism and a pale to eosinophilic cytoplasm, some of which showed an intracytoplasmic vascular lumen (Figure 1D). These cells displayed positive immunophenotyping for CD31, CD34, and factor VIII related antigen stains with ki67 proliferation index of 5%.
A perihepatic artery lymph node was found to be involved by metastatic disease at the time of surgery. The patient has been under treatment with sirolimus immunosuppressant ever since, with no evidence of tumor progression.
2.3. Case 3
A 53-year-old woman with a history of chronic hepatitis B went under an orthotopic liver transplantation for a relapse of HEHE at our center in March 2018. She had a history of partial hepatectomy in March 2016. After being diagnosed with HEHE in January 2016, two liver masses and an aortocaval space mass were resected and confirmed as EHE in pathologic examination. On gross examination of the explanted liver, a total of four masses were found involving both right and left liver lobes, with the largest mass measuring 5 cm in greatest dimension.
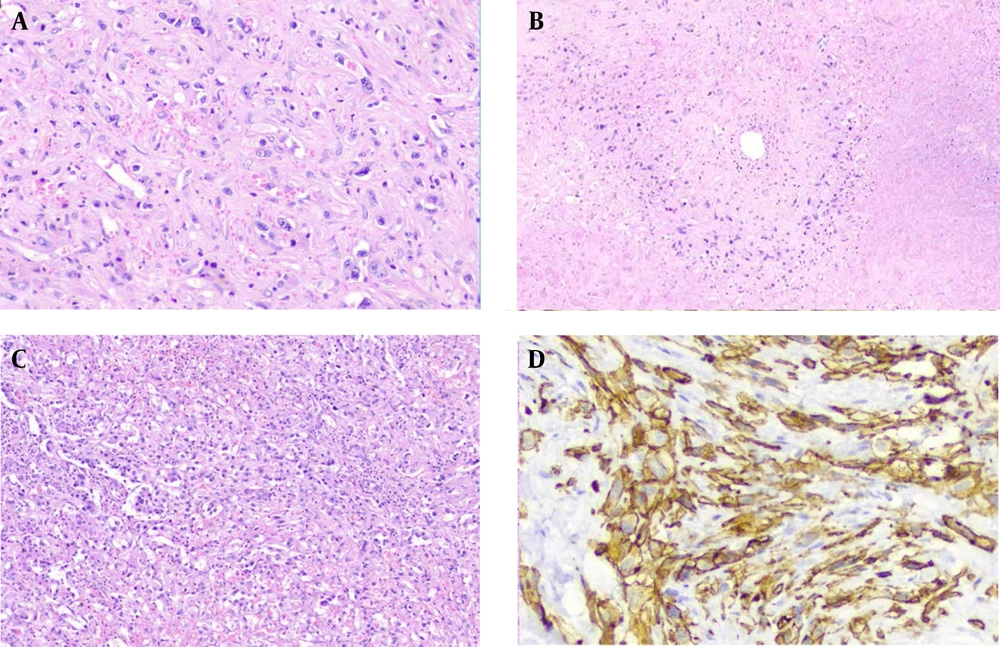
Microscopically, the tumor showed extensive necrosis and hemorrhage, among which a hyalinized myxoid matrix was identifiable (Figure 2B). This matrix enclosed tumor cells with a spindle and epithelioid morphology. Some of these cells displayed intracytoplasmic spaces containing red blood cells (Figure 2A). Interestingly, areas of marked cellularity with noticeable atypical nuclei were seen (Figure 2C). The immunophenotyping with vascular markers such as CD34 confirmed the endothelial nature of the tumor (Figure 2D). Ki67 was expressed in about 10% of the tumor cells. Two years after liver transplantation, the tumor relapsed once again, this time at the right pleura. No surgical intervention has been done since, and the patient is still alive and under observation as this report is being written.
(A) Myxohyaline stroma embedding tumor cells, some with intracytoplasmic vascular spaces filled by red blood cells (H&E, x400). (B) The infiltrating tumor cells are surrounded by a necrotic and hyalinized matrix at the periphery. A central vein residue is seen in the center of the picture (H&E, x100). (C) Area of the tumor showing marked cellularity with a moderate degree of atypia (H&E, x100). (D) Positive membranous staining with CD34 (x400).
3. Discussion
Since the description of EHE in the soft tissue by Weiss and Enzinger nearly 38 years ago, this neoplasm has been recognized in other organs, including the lung, liver, mediastinum, and bone (6, 7). Ishak was the first to report EHE in the liver, studying the clinical, morphologic, and follow-up of HEHE in a series evaluating 32 patients in 1984 (8). In the coming years after his report, many studies have addressed this rare tumor from different perspectives. As its name implies, HEHE is a vascular tumor in nature, originating from endothelial cells, yet resembling an epithelioid neoplasm. This tumor has a low incidence rate of less than 0.1 per 100,000 (7) It is more likely seen in women with a female-to-male ratio of 3:2 (7); although it presents in a wide age range, it is more likely seen in middle-aged adults with a mean age of 41.7 years(4) The predisposing factors of HEHE are not known, although oral contraceptives, primary biliary cirrhosis, hepatitis B virus, alcohol, and substance exposure to vinyl chloride, polyurethane, silicone, asbestos, and thorotrast are known as possible risk factors (4). The presenting clinical symptoms, signs, and laboratory findings are usually nonspecific, and the tumor may be found incidentally on imaging studies in an asymptomatic patient (9, 10) Among symptomatic patients, right upper quadrant pain, hepatomegaly, and weight loss are found to be the most common complaints (4), but more pronounced manifestations such as portal hypertension, Budd–Chiari syndrome, or hepatic failure are also observed (11). Tumor markers such as carcinoembryonic antigen (CEA), CA19-9, and alpha-fetoprotein are usually in the normal range (12).
Multicentricity with the involvement of both liver lobes is the general presentation of this tumor, but it can also be seen solitary in a minor population (4). At its early stages, it appears as discrete nodules in different sizes ranging from 0.5 to 12 cm, which is commonly located at the liver periphery and invading the capsule (12). Later, as these nodules grow in size, they form complex and confluent masses, which eventually coalesce and form a diffuse pattern. Accordingly, radiologic findings of HEHE may differ based on the stage of the tumor. HEHE nodules, in general, show a low attenuating center surrounded by a peripheral rim of enhancement on contrast-enhanced CT scan and magnetic resonance imaging (MRI) images (3). One clue to the diagnosis in such images is the retraction or flattening of the liver capsule caused by fibrosis (4, 12). The ‘halo sign’ and the ‘lollipop sign’ are also described as the imaging features of HEHE (7). Intra-tumoral calcification is also a helpful diagnostic feature of this tumor found in 15-25% of cases, which is best appreciated on CT scan (3) In Mehrabi’s meta-analysis on HEHE, 36.6% of patients showed evidence of extrahepatic involvement at initial diagnosis, with the lung being the most common site of metastasis, followed by the regional lymph nodes, peritoneum, bone, spleen, and the diaphragm, respectively (4). Interestingly, metastatic spread to other organs at the time of diagnosis does not appear to change the patient prognosis, considering the favorable reports of long-term disease-free survival rates after liver transplantation (4, 5, 13). Occasionally, the tumor advances into the surrounding structures and may cause portal hypertension as a result (7).
On gross examination, a nodular or diffuse pattern is seen depending on the tumor stage. The tumors nodules are ill-defined with a firm consistency and a white gritty cut surface looking similar macroscopically to cholangiosarcoma (14).
Histologically, contrasting its nature, this tumor does not show a recognizable pattern of vascular canalization characteristic of vascular tumors (14). The tumor infiltration of HEHE has a somewhat zonal distribution. The periphery of the tumor is more cellular with less of a stroma component, yet the center of the tumor is composed of a hypocellular sclerotic, myxohyaline, or chondroid stroma with necrosis, and at times calcification. The tumor cells are composed of three types of cells, including the main round epithelioid cells, the spindled or stellate dendritic cells which are dispersed among the main cells with their cytoplasmic processes, and the intermediate cells with the features of both the epithelial and dendritic cells (7). These tumor cells infiltrate the liver parenchyma in a single cell or cord-like growth pattern, invading the sinusoids and using them as a platform, destroying the hepatocytic plates, vessels, and the sinusoids on their way. Despite all this tumor obliteration, the lobular architecture is still identifiable with visible remains of the portal tracts and central veins. Occasionally, intracytoplasmic vacuoles containing red blood cells are seen in the tumor cells giving them an intracellular small capillary lumen-like appearance (14). At times, these vacuoles give the neoplasm a signet ring cell look but with no mucin content. The tumor cells show mild atypia and pleomorphism. Mitosis is variable, but it is usually rare or absent. Tumor growth into the sinusoids and portal or hepatic veins causes tuft-like proliferations that not only simulate an intravascular invasion of carcinoma, but also are responsible for hepatic outflow obstruction, which may lead sometimes to Budd Chiari syndrome.
Endothelial immunohistochemical (IHC) markers such as CD34, CD31, factor VIII related antigen, FLI-1 protein, and podoplanin (D2-40) help in acknowledging the true identity of these epithelial looking tumors as vascular. Among such markers, podoplanin is a lymphatic vascular immunostain more commonly expressed in liver EHE and is more specific for it (7, 9). Fujji et al. showed that podoplanin is expressed much more in liver EHE compared to other vascular tumors, such as angiosarcoma, sclerotic hemangioma, angiomyolipoma, cholangiosarcoma, and hepatocellular or metastatic carcinoma, with a 78% sensitivity and 100% specificity (15). FLI-1 protein is also a much more sensitive endothelial marker in identifying HEHE explicitly, with a high specificity (7). In contrast, CD34 stains 90% of different vascular tumors and thus is not a specific marker for HEHE (7). The most commonly identified genetic abnormality in EHE is CAMTA1-WWTR1 fusion protein resulting from the translocation t(1;3) (p36.3;q25) (9). This fusion product appears to be specific for HEHE and negative in epithelioid angiosarcoma or other potential tumor mimickers, and thus can be used as a tumor marker for diagnosis of EHE (8, 16). Nuclear expression of ERG vascular endothelial marker is also very specific (17).
Not uncommonly, the histopathologic features of HEHE are misdiagnosed with angiosarcoma, cholangiocarcinoma, hepatocellular carcinoma (HCC), in particular the sclerosing variant, metastatic carcinoma, and sclerotic hemangioma (7, 9, 10). IHC staining for cytokeratin helps to differentiate mimickers with true epithelial nature including HCC, cholangiocarcinoma, and metastatic carcinoma. It is worth mentioning that cytokeratin or epithelial membrane antigen (EMA) staining entrapped bile ducts or nontumoral hepatocytes can be seen in HEHE and should not be considered as tumor positivity. On the other hand, CD34 may be positive in HCC, but in contrast to the diffuse staining of tumor cells in HEHE, it is only expressed in continuous with the sinusoids and not the neoplastic cells. HEP par1, arginase, and canalicular CD10 positive immunostaining are features of HCC and not HEHE (7). Angiosarcoma, in particular, can be confusing since, as a vascular tumor, it shares with HEHE similar histologic features and IHC staining for factor VIII, CD31, and CD34. Unlike HEHE, angiosarcoma is a more architecturally destructive tumor with an inter-anastamozing histologic pattern, prominent atypia, and a higher mitotic count, yet with less sclerosis than EHE (7, 18).
HEHE has an astonishing variable natural course, ranging from an indolent tumor with a prolonged survival, even in the absence of therapeutic intervention, to an aggressive, fatal neoplasm (5). Indeed, localized liver EHE may develop recurrence and metastases in a short term after liver transplantation, whereas those cases with metastatic disease at the time of the diagnosis have been seen with long-term disease-free survival (4).
Reliable histologic parameters useful in predicting the aggressiveness of HEHE have not yet been met. Studies conducted in approaching this matter have presented increase in cellularity and micro- or macro-vascular invasion as valuable factors that correlate with poor clinical outcome (11, 13).
The degree of cellular pleomorphism, proliferation activity, necrosis, Glisson’s capsule invasion, and tumor size has long been argued as incapable factors in predicting the behavior of HEHE (11). Yet cases manifesting a high ki-67 proliferation index and cellular atypia have been reported to show a poor clinical course, suggesting such parameters as indicators of aggressive behavior (19).
A large cohort survey by Lai et al. conducted on the European Liver Transplant Registry presented macrovascular invasion, a short waiting time (≤ 120 days), and hilar lymph node involvement as risk factors for post-liver transplant recurrence (13). In their study, metastatic disease before liver transplantation was not found as a risk factor for post-surgery recurrence (13). Based on these findings, they created an HEHE liver transplantation score not only to estimate the risk of tumor recurrence, but also as a means for the type of adjuvant therapy used in patient management.
From the early days of its recognition to date, there is still no single guideline on how to treat HEHE. This may be explained by the unpredictable nature of this tumor and various outcomes of different therapeutic strategies. A broad range of treatment approaches have been used with different outcomes, including partial hepatectomy, chemoradiation, tumor ablation, and liver transplantation or only pure observation without any therapeutic intervention (7, 9).
Liver transplantation has been the most common treatment approach with a better 5-year survival rate (82%), and a low recurrence rate (36.4%) in comparison to other strategies, mostly because HEHE manifests as multiple tumors involving both lobes making it unresectable by partial hepatectomy (4). It is also a justified treatment for patients with coexistent extrahepatic tumor metastasis (20). Such patients have shown improvement in symptoms with relative stability of the extrahepatic metastatic disease after receiving liver transplantation (4).
In rare cases of focal respectable HEHE, partial hepatectomy is a treatment choice with a high rate of survival, even though an aggressive course has been seen after palliative tumor resection, including minor and major hepatectomies (4, 13). Enhancement of tumor growth under immunosuppression therapy and the insufficiency of an effective post-surgical adjuvant chemotherapy are the proposed factors in causing tumor recurrence (4).