1. Background
Viral hepatitis C represents a major global public health concern which disproportionately affects people who use drugs (PWUD) (1, 2). The World Health Organization (WHO) has set a target to eliminate hepatitis C (HCV) as a public health threat by 2030, with a goal to reduce new HCV infections by 90% and associated deaths by 65% (3). Injection drug use is the most common mechanism of incident HCV transmission in higher income countries (3). We must accelerate the treatment of PWUD to meet WHO elimination targets (4). Treatment delivery and initiation among PWID has been challenged by social, structural and policy barriers. Many health systems, particularly in the United States, limit treatment in this high-risk population, often citing inadequate outcomes data (5-8).
There is a growing body of literature showing similar sustained viremic response (SVR), or cure, of hepatitis C among PWID as compared to those who do not use drugs (9). Much of the literature focuses on treatment among people on medications for opioid use disorder, such as buprenorphine or methadone. There are less data available for HCV treatment among people engaging mostly with harm reduction services. Modelling studies suggest that treating PWID who are less engaged in traditional health care settings will be necessary to meet elimination targets (4).
2. Objectives
Our aim was to compare outcomes of treatment of chronic hepatitis C in PWID enrolled in a community MOUD program or a syringe services program (SSP) to continue to develop our understanding of treating hard to reach populations.
3. Methods
3.1. Study Design and Setting
In this real-world, multi-site prospective open-label trial, we enrolled PWID (defined as people with current or previous injection drug use) with confirmed HCV viremia from two distinct clinical sites. The MOUD group were enrolled from Old Town Clinic, a healthcare for the homeless clinic in Portland, Oregon that provides wrap around multidisciplinary services to individuals living with homelessness and substance use disorders. Participants in the SSP group were enrolled from the Outside In SSP, a nearby social services agency that offers harm reduction services in an associated federally qualified health center medical clinic. Eligible participants were offered elbasvir/grazoprevir 50mg/100mg (E/G) orally for 12 weeks. These prospective cohorts were compared to a retrospective cohort of patients seen in an academic hepatology clinic (AHC) who received treatment with E/G over the same time period as the MOUD and SSP groups were enrolled. This study was designed to assess efficacy of screening and treatment within MOUD treatment programs as compared to SSPs.
This study was approved by the Oregon Health & Sciences University Institutional Review Board. Informed consent was collected on all participants in the prospective trial. The retrospective community control group analysis was performed without individual consent, in accordance with institutional review board guidance. All research activities confirmed to the ethical guidance of the 1975 Declaration of Helsinki. This trial was registered with clinicaltrials.gov, NCT03093415.
3.2. Participants
Participants in the prospective trial were eligible to participate if they were at least 18 years of age; current or previous history of injection drug use; confirmed HCV viremia and genotype 1b or 1a without baseline NS5a (nonstructural protein 5a) resistance-associated variants (RAVs); low fibrosis, defined as an Aspartate Aminotransferase Platelet Ratio Index (APRI) of less than 0.7 (10-12) or transient elastography (FibroScan) or Fibrosure suggesting metavir fibrosis score of F2 or less; and ability to make two of three sequential office visits. Participants were excluded if there was laboratory or clinical evidence of cirrhosis; elevated prothrombin time unrelated to anticoagulation, hemoglobin level less than 12.3g/L in females and less than 14g/L in males, platelet count < 150 cells × 109/L, white blood cells (WBCs) < 4.0 × 103/mm3, aminotransferase levels more than 10 times the upper limit of normal, or albumin levels < 3.5 g/L; previous treatment for hepatitis C infection; known hepatocellular carcinoma; human immunodeficiency virus (HIV) or hepatitis B virus co-infection; pregnancy, or contraindicated drug interactions (i.e., strong CYP3A inducers) with elbasvir-grazoprevir and inability to change regimens to avoid interaction. As this study was designed to assess the role of the treatment site rather than individual exposures, participants in the MOUD group were not excluded if they engaged with an SSP, and those in the SSP group were not excluded if they were taking MOUD.
The AHC retrospective comparison group analysis employed the same inclusion and exclusion criteria as the prospective sites, except for the requirement for a history of injection drug use due to missing data.
3.3. Procedures
Enrolled participants received a fixed dose elbasvir 50 mg and grazoprevir 100 mg for 12 weeks. We assessed participants at screening, enrollment, weeks 2, 4, 8, and 12 of therapy, and 12 weeks after treatment completion (SVR12). Assessments were done at the time of dispensation of E/G medications during treatment and during health services visits for week 12 and 24 assessments. Reinfection screening assessment at 48 weeks post treatment initiation was also planned but not completed in the SSP group due to study staff limitations.
Screening and enrollment assessments included HCV RNA and genotype with NS5a resistance testing, APRI and Fibrosure or Fibroscan for determination of baseline fibrosis, and hepatitis A and B serologies. Substance use, MOUD and syringe services, and comorbid mental health disorders were also assessed at baseline. HCV-associated stigma questionnaire was performed in all participants and 25 participants were offered participation in structured qualitative interviews, published previously (13). Medications were dispensed at enrollment and weeks 2, 4, and 8 of treatment in individual pill bottles separate from other medications. E/G adherence (self-report confirmed with left over pills at study pharmacist visit), active substance use, MOUD adherence, and SSP utilization were collected at all follow up visits. Fifteen dollars incentives were given for participation in enrollment, surveys, and qualitative interviews, but not medication adherence or SVR12 assessments.
3.4. Outcomes
Our primary outcome was intention-to-treat sustained viremic response at 12 weeks after completion of treatment (ITT SVR12). ITT SVR12 was defined as a confirmation of undetectable HCV RNA at least 12 weeks after the end of treatment among participants who initiated E/G. This ITT SVR12 definition was based on previous literature in a similar population (14, 15). Prespecified secondary outcomes were modified per protocol SVR12 (mPP SVR12), treatment discontinuation, good medication adherence (defined as greater than 90% pills taken), and percentage of participants with NS5a RAVs at screening. Modified per protocol SVR12 was defined as those with undetectable HCV RNA 12 weeks after end of treatment, among those initiating E/G who completed SVR12 confirmation labs.
3.5. Statistical Analysis
Based upon a sample size of n = 50 subjects in the combined safety net/needle exchange clinics and 50 subjects in the retrospective comparator group, this study has 80% power to detect a 20% difference between groups; this calculation is based on the assumption that the retrospective comparator group will have and SVR12 rate of 95% (taking into account treatment discontinuation and relapse rates, i.e., intention to treat analysis) compared to SVR12 rate of 75% in the safety net clinic patients.
Patient baseline characteristics were summarized according to study site. Continuous parametric variables were compared using Student’s t-test and categorical variables using the chi-squared test. Binary logistic regression was performed to identify independent variables predicting the primary outcome. Statistical analysis was performed using SPSS 26 (SPSS Inc., Chicago, Illinois) statistical package.
4. Results
4.1. Participants
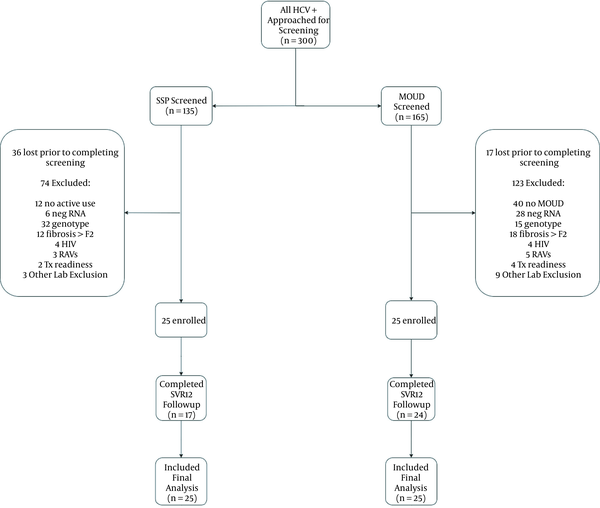
Of the 300 participants screened for the MOUD and SSP groups, 250 were excluded, most commonly for incomplete phlebotomy for screening (53/300; 18%) or wrong genotype (47/300; 16%) (Figure 1). Only 3% (8/300) were excluded due to RAVs inconsistent with the study medication. Fifty participants were enrolled between May 30, 2017 and June 6, 2018, and received elbasvir/grazoprevir therapy (Table 1; Figure 1). The MOUD and SSP groups were similar in age, sex, race, and APRI score. By design, all in the SSP group injected drugs within the last month and all in the MOUD group were on buprenorphine (11/25; 44%) or methadone (14/25; 56%). In the MOUD group, 9/25 (36%) also reported at least one engagement with the SSP during treatment, and 14/25 (56%) in the SSP group were also on MOUD, 6 of whom (43%) started MOUD during the study period. The AHC group was significantly older and had a higher APRI score than the two prospective groups. Only 13/50 (26%) in the AHC group carried substance use diagnoses; active use data were not collected.
| Variables | MOUD (n = 25) (%) | SSP (n = 25) (%) | AHC (n = 50) (%) |
|---|---|---|---|
| Age (y) a | 44 (32 – 54) | 41 (29 – 53) | 59 (52 – 67) |
| Sex b | |||
| Male | 15 (60) | 15 (60) | 33 (66) |
| Female | 10 (40) | 10 (40) | 17 (34) |
| Race/ethnicity b | |||
| White | 22 (88) | 22 (88) | 50 (100) |
| Black/African American | 1 (4) | 1 (4) | 0 (0) |
| First nations/indigenous | 1 (4) | 0 (0) | 0 (0) |
| Asian/pacific islander | 1 (4) | 0 (0) | 0 (0) |
| Other/declined | 0 (0) | 2 (8) | 0 (0) |
| Housing status b | (No Data) | ||
| Houseless/unstable | 4 (16) | 8 (32) | |
| Stable/transitional | 21 (84) | 17 (68) | |
| Established primary care b | (No Data) | ||
| Not established | 0 (0) | 4 (16) | |
| < 1 year | 6 (24) | 7 (28) | |
| > 1 year | 19 (76) | 14 (56) | |
| Baseline genotype b | |||
| 1a | 22 (88) | 24 (96) | 36 (72) |
| 1b | 3 (12) | 1 (4) | 14 (28) |
| 4 | 0 (0) | 0 (0) | 0 (0) |
| APRI score b | |||
| < 0.7 | 19 (76) | 23 (92) | 30 (60) |
| > 0.7 | 6 (24) | 2 (8) | 20 (40) |
| Drug of choice b | (No Data) | ||
| Heroin | 23 (92) | 16 (64) | |
| Methamphetamine | 0 (0) | 9 (36) | |
| Alcohol | 1 (4) | 0 (0) | |
| Cannabis | 1 (4) | 0 (0) |
Baseline Characteristics
4.2. Assessing SVR12
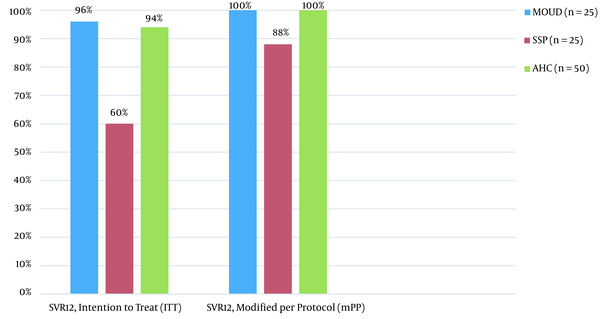
In intention to treat (ITT) analysis, 24/25 (96%) in the MOUD and 15/25 (60%) achieved SVR12, which was statistically significant (P < 0.0001). There were two confirmed treatment failures, both in the SSP group, with 24/24 (100%) of the MOUD group and 15/17 (88%) of the SSP group achieving SVR12 in modified per protocol analysis (Figure 2, Table 2).
| Variables | MOUD (n = 25) | SSP (n = 25) | AHC (n = 50) | Statistical Significance a |
|---|---|---|---|---|
| SVR12, intention to treat (ITT) | 96% (24/25) a | 60% (15/25) a | 94% (47/50) | < 0.0001 |
| SVR12, modified per protocol (mPP) | 100% (24/24) a | 88% (15/17) a | 100% (47/50) | 0.014 |
| Adherence greater than 90 % pills taken | 100% (25/25) a | 68% (17/25) a | (No Data) | 0.003 |
| Treatment discontinuation < 4 weeks completed | 0% (0/25) a | 36% (9/25) a | 6% (3/50) | < 0.0001 |
Primary and Secondary Outcomes
4.3. Assessing Secondary Outcomes
When combined, 41/50 (82%) completed treatment in the SSP and MOUD cohorts (defined as taking > 90% total regimen), 25/25 (100%) and 17/25 (68%) in the MOUD and SSP groups, respectively. Adherence and treatment completion rates were unknown in the AHC comparison but presumed by filling last prescription 50/50 (100%). Participants on MOUD, Adherence > 90%, and those completing therapy were all statistically more likely to achieve ITT SVR12 (Table 3). Housing status and recent drug use was not associated with SVR12.
| Variables | SVR12, ITT, % (n) | Odds Ratio (OR) | 95% Confidence Interval | Statistical Significance |
|---|---|---|---|---|
| Adherence | 11.24 | 1.88 - 53.23 | 0.01 | |
| Greater than or equal to 90% pills taken | 86 (36/42) | |||
| < 90% pills taken | 38 (3/8) | |||
| Last drug use | 5.6 | 0.65 - 48.42 | 0.129 | |
| Greater than 6 months | 93 (14/15) | |||
| Less than 6 months | 71 (25/35) | |||
| MOUD use | 4.58 | 1.05 - 19.96 | 0.043 | |
| Yes | 85 (33/39) | |||
| No | 55 (6/11) | |||
| Housing status | 3.93 | 0.45 - 34.43 | 0.257 | |
| Houseless/unstable | 92 (11/12) | |||
| Stable/transitional | 74 (28/38) | |||
| Treatment discontinuation | 7.29 | 1.51 - 35.20 | 0.01 | |
| Yes | 44 (4/9) | |||
| No | 85 (35/41) |
Predictors of SVR12 (MOUD and SSP Groups) by Logistic Regression
4.4. Adverse Events
At least one minor side effect was noted by 33/50 (66%) of patients in the prospective arms and more commonly reported in the SSP than MOUD group, 18/25 (72%) vs 15/25 (60%), respectively. Headache, nausea and fatigue were most commonly reported. No side effects led to medication discontinuation.
5. Discussion
This prospective real-world study adds nuance to the literature around the challenges of (a) maintaining people on HCV treatment and (b) confirming cure after treatment completion in an SSP setting.
We observed significantly higher intention-to-treat SVR12 rates in those receiving treatment in the MOUD (96%) versus SSP setting (60%). These differences were driven mostly by nonadherence to SVR12 lab visits, as the per protocol SVR12 rate was more similar at 100% vs 88%, respectively, though this remained statistically significant (P = 0.01). Adherence was generally good in both arms, though higher treatment discontinuation rates were seen in the SSP arm. Treatment was generally well tolerated with mild side effects, none of which led to treatment discontinuation. The community standard academic hepatology group was comparatively older, with more socioeconomic stability, achieving a similar SVR12 rate to the MOUD group.
The MOUD group performed similarly to average cure rates in real world studies among people who use drugs, (16-18) while the ITT cure rates were slightly lower in the SSP group than average rates reported in the meta-analysis by Hajarizadeh et al. (9). The lower ITT SVR12 rate in the SSP group likely reflects multiple factors. We financially incentivized study enrollment but intentionally did not incentivize engagement with study visits, medication pickups, or SVR12 lab results. The SSP group also had a higher rate of homelessness and other factors previously associated with lower SVR12 rates, especially in ITT analysis, driven by completion of lab results (18-20). Our data did not show homelessness to be negatively associated with SVR12, though it was inadequately powered for this association and other data our group has published has found lower SVR12 completion among homeless individuals (21). For many PWID, especially those who experience homelessness, personal cost-benefit analysis of completing lab work after finishing a medication with high chances of cure are seen by some participants to be less favorable than more immediate concerns (22). People who use opioids must often prioritize procuring opioids to avoid withdrawal. This need may be less pronounced for those on MOUD, which prevents opioid withdrawal. The disproportionate number of people actively using opioids in the SSP group may have affected these participants’ willingness to complete study labs, and the lack of telephone access may have contributed to less SVR12 lab completion in the SSP group. In addition, real-world, non-incentivized studies have been shown to have lower SVR12 lab completion rates than financially incentivized prospective clinical trials among PWID (9). Implementing financial incentives to routine HCV treatment in this population may improve engagement and lab completion.
It should be noted that while low adherence was associated with low ITT SVR12 rates, treatment discontinuation was most strongly associated with lower ITT SVR12. The actual cure rate is difficult to assess in the SSP group given the substantial portion who did not return for SVR12 lab results. While interesting that our study showed lack of MOUD and low adherence to be negatively associated with SVR12, the degree to which the SSP participants did not complete SVR12 labs demonstrates mostly the willingness to complete labs as opposed to rate of cure. This may in part explain why our results differ from those of other larger, better funded studies, such as PREVAIL and SIMPLIFY, which did not show significant impacts of substance use or adherence (15, 23). These studies also employed electronic blister packs and were better suited to describe the relationship between adherence and cure.
Perhaps the most important finding from our study is found in a review of screening data (Figure 1). Out of a total of 300 participants with chronic HCV screened, 250 were unable to make it to enrollment. A total of 82 were excluded by investigator driven constraints, such as inclusion criteria requiring low fibrosis status, or MOUD or active drug use. However, several factors related to the study drug E/G also contributed. Fifty three patients were excluded were due to inability to complete the high volume of lab work necessitated by this agent (notably genotype and NS5A resistance testing), 47 due to wrong genotype (exclusively genotype 3), and 8 due to RAVs inconsistent with the study drug. This reflects a total of 36% of the overall screened population. In a clinical environment the participants with genotype 3 HCV would often be offered other treatment options, but the high burden of lab testing would remain. Pan genotypic regimens, streamlining lab orders to limit volume, and non-phlebotomy confirmation tests such as dried blood spot may all lower barriers to treatment uptake in this population.
Our study had several important limitations. First, the two study groups were separated primarily by site of clinical engagement (MOUD and SSP settings), though there was crossover exposure to both MOUD and SSP engagement at both sites. While our data allow some comparisons of HCV treatment outcomes from these different clinical settings, our ability to assess the role of MOUD or SSP engagement on HCV treatment outcomes is limited. Additionally, the small sample size was powered sufficiently to detect a difference in the primary outcome between recruitment sites but not to detect meaningful differences in individual predictors of participants completing treatment or achieving SVR12.
5.1. Conclusions
This study adds depth and nuance to the existing literature on treatment of chronic HCV in people who use drugs. Our data show that people who use drugs can be successfully treated with DAAs, though those with continued drug use may struggle to complete lab work confirming HCV cure. Extensive pre-treatment laboratory evaluation – especially when using medications requiring genotype and NS5a resistance testing – remains a barrier to treatment. More research is needed into simplified pre-treatment evaluation, use of pangenotypic regimens, and incentivizing SVR12 lab completion to address these barriers to hepatitis C elimination among people who use drugs.
6. Role of the Funding Source
This study (including study drugs) was funded through an investigator-initiated grant by Merck & Co., Inc. The funder had no role in the analysis or interpretation of results. All listed co-authors had access to the raw data but these were not shared with or requested by the funder. AZ and AS were responsible for the statistical analysis, manuscript development, and decision to submit for publication.