1. Background
Hepatitis C virus (HCV) is a global health threat that more than 71 million individuals have been estimated to be infected with worldwide (1). After infection, 80% - 85% of the infected individuals become persistently infected and mostly lead to hepatic fibrosis, cirrhosis, and hepatocellular carcinoma (2). Although direct-acting antivirals (DAAs) have been shown to cure the infection (3), the treatment effect of different genotypes is different, and the virus resistance is increasing.
It was reported that a genotype 2a strain of HCV, JFH-1, can efficiently replicate and generate infectious HCV in the Huh7 cell line (4, 5). This in vitro culture system of HCV provides a powerful tool for studying the viral life cycle and developing antiviral strategies (6). The purification of HCV is very important for its intensive research. However, ultracentrifugation was the only traditional method; however, its application is limited due to being time-consuming and requiring expensive technical equipment. Additionally, ultracentrifugation could not separate the virus from other contaminants with similar densities and is not suitable for large-scale purification. Therefore, more effective and convenient methods for HCV purification are needed.
Heparin is a glycosaminoglycan observed in the secretory granules of mast cells (7) and basophils (8). Heparin interacts specifically with proteins (9, 10), such as recombinant HCV envelope protein 2 (E2) (11). It was reported that HCV particles from infected human plasmas could be purified by heparin-affinity chromatography (12); nevertheless, it has not been reported in JFH-1 cultures.
The magnetic separation technique has become an important and common approach for sample purification in biological analysis, with advantages of simple operation, good biocompatibility, and high binding capacity. These have been widely applied in various separation processes of protein/peptide, nucleic acid, cell, bioactive compound, and enzyme immobilization (13-15). Only polyethylenimine (PEI) beads have been used for HCV concentration (16). However, it has no accurate selectivity and cannot be considered an effective purification process for virus purification. The affinity of biotin for streptavidin is one of the strongest and most stable interactions in biology. The combination of the two experimental methods may develop a new technique for HCV purification.
2. Objectives
The purification of HCV is a very crucial step for further study. However, as the only traditional method, ultracentrifugation cannot be widely used because it needs special instruments. Therefore, more effective and convenient methods for HCV purification are necessarily needed. To solve this problem, the present study developed two alternative simple and effective purification methods using heparin-affinity chromatography and magnetic separation techniques.
3. Methods
3.1. Cell Culture and Establishment of Serum-Free Culture Cells
Human hepatoma cell line Huh7.5.1 was kindly provided by Yuanping Zhou (Department of Infectious Diseases, Nanfang Hospital, Guangzhou, China). The cells were cultured at 37°C in Dulbecco’s modified eagle medium (DMEM) containing 10% fetal bovine serum (FBS) under the condition of 5% CO2. For serum-free culture, the cells were conditioned and cultured in Dulbecco’s modified eagle medium/nutrient mixture F-12 supplemented with insulin-transferrin-selenium-X (ITS) (Life Technologies Corp., Grand Island, NY, USA). The Huh7.5.1 cells were sequentially passaged in DMEM containing 10%, 5%, and 1% FBS and ultimately into the serum-free media containing ITS every 3 days.
3.2. JFH-1 Synthesis and Transfection
Plasmid pJFH-1 was an HCV consensus clone (genotype 2a) derived from a Japanese patient with fulminant hepatitis, kindly provided by Yuanping Zhou (Department of Infectious Diseases, Nanfang Hospital, Guangzhou, China). The pJFH-1 was linearized by XbaI digestion and transcribed into ribonucleic acid (RNA) under T7 promoter in vitro using the T7 MEGAscript Kit (Ambion/Applied Biosystems, Austin, TX, USA). For the generation of JFH-1 virus particles, RNA was transfected into Huh7.5.1 cells by Lipofectamine 2000 (Invitrogen) at an RNA lipofectamine ratio of 1:2 using 5 µg of JFH-1 RNA in 104 cells. The cells were passaged every 3-5 days. The production of HCV in these cells and corresponding supernatants were detected at the indicated time points.
3.3. RNA Extraction and RT-qPCR
Viral RNA was extracted from the supernatant of transfected cells using the High Pure Viral Nucleic Acid kit (Roche Diagnostics GmbH, Mannheim, Germany). Quantification was performed using reverse transcription-quantitative polymerase chain reaction (RT-qPCR) with primers targeting HCV 5’NCR (5’-tgctagccgagtagygttgg-3’, 5’-actcgcaagcaccctatcag-3’, 5’-(FAM) accacaaggcctttcgcgac (BHQ-2)-3’).
3.4. Infectivity Assay
The infectivity of the culture medium from RNA-transfected cells was determined by end-point dilution assay and immunofluorescence (13). In this study, 100 µL of serially 5-fold diluted samples in DMEM medium was added to at least six wells per dilution and incubated with Huh7.5.1 at 37°C for 4 h. Infection titer was examined 72 h after inoculation by immunofluorescence using a Mouse Monoclonal Anti-HCV Core C7-50 (Abcam, Cambridge, UK) and the Alexa Fluor 594-Conjugated Goat Anti-mouse Secondary IgG (H + L) (Invitrogen China Limited, Guangzhou, China). The infectious foci were calculated and expressed as focus forming units per milliliter (FFU/mL).
3.5. HCV Production and Concentration
After transfection, the culture medium was collected, and fresh serum-free media was added to the cells. Then, culture medium collection was repeated every 3 - 4 days after cell passage until virus production decreased. After low-speed centrifugation, the culture medium was passed through a 0.45 µm filter and concentrated approximately 50 folds using Centricon® centrifugal filter devices with a 100 kDa cut-off (Amicon®, Merck Millipore, USA) at 3000 rpm at 4°C.
3.6. Heparin-Affinity Chromatography
Concentrated cell cultures containing HCV particles were equilibrated to 0.05 M Tris (hydroxymethyl) aminomethane (THAM) hydrochloride (Tris HCl), prepared for heparin-affinity chromatography as previously described (12). Briefly, unbound segment proteins were removed by 0.2 M sodium chloride (NaCl) equilibration buffer (EB), and the bound segment was eluted with 0.3 and 0.4 M NaCl EB. Each elution fraction was concentrated using Centricon® centrifugal filter devices with a 100 kDa cut-off (Amicon®, Merck Millipore, USA) at 3000 rpm at 4°C.
3.7. Magnetic Separation Technique
A series of serum samples from HCV-infected blood donors were kindly provided by Maoming Blood Center (Guangdong, China), with specific antibodies confirmed by two commercial enzyme-linked immunosorbent assay kits. The western blot with JFH-1 cell lysate was performed to determine whether high levels of anti-HCV antibody were present. Sample MM110 was chosen through detection due to its high levels of anti-HCV E2 antibody and extremely low levels of anti-HCV core antibody. This blood donor was male, 43 years old, anti-HCV antibody positive (S/CO = 15.95), with 4.0 × 104 IU/mL of HCV RNA load (genotype 1b) and 72.47 U/mL of alanine aminotransferase level in plasma. The antibodies were purified by protein A (HiTrap rProtein A FF 1 mL) and biotinylated antibodies using BiotinTagTM Micro Biotinylation Kit (Sigma-Aldrich, USA).
Concentrated cell culture supernatants with HCV particles were pre-incubated with the biotinylated antibody at 37°C for 2 h and then purified using MagneSphere® magnetic separation products (Promega Corp., WI, USA) with the typical protocol to capture the biotinylated antibody in MM110 using the Streptavidin MagneSphere® Paramagnetic Particles (SA-PMPs). The reaction requires 1 mg of antibody and 10 ml of SA-PMPs (at 1 mg/mL). The virus was eluted by 0.1 M sodium citrate (pH = 3) and immediately neutralized by 1 M Tris HCl (pH = 9.0).
3.8. Western Blot
The purified viruses or Huh7.5.1 cell lysates were electrophoresed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (MilliporeSigma, Massachusetts, USA). The protein-bound membranes were incubated with a Mouse Monoclonal Anti-HCV Core C7-50 (Abcam, Cambridge, UK), detected by Goat Anti-Mouse IgG and IgM HRP Conjugate, and visualized by adding immunochemiluminescence reagent (ECL, Millipore, Billerica, Massachusetts, USA). The naïve and JFH-1 transfected Huh7.5.1 cells were used as negative and positive controls, respectively. In quantifying HCV antibody levels in the serum sample from HCV infected blood donors with transfected Huh7.5.1 cells, the serum was used as the primary antibody and detected by Goat Anti-human IgG and IgM HRP Conjugate.
3.9. Electron Microscopy and Immune-Electron Microscopy
The concentrated and purified viruses were adsorbed onto carbon-coated grids for 10 min. Then, the grids were negatively stained with 2% aqueous sodium phosphotungstate for 3 min. After a quick wash with distilled water, the grids were examined with a Hitachi H-7650 electron microscope. For immunogold labeling, the virus particles were first incubated with anti-HCV E2 (AP33) (14) for 2 h at 37°C, diluted 1:20 in phosphate-buffered saline (PBS). The JFH-1 viruses were further incubated for 1 h at 37°C with Anti-Mouse IgG (whole molecule)-Gold (10 nm in diameter; Sigma-Aldrich, USA), diluted 1:20 in PBS. After washing, negative staining of the viruses was performed as described above. An anti-brucellosis bp26 antibody was used as a negative unrelated primary antibody of HCV (15).
4. Results
4.1. Sustained Virus Production in Serum-Free Cultured Huh7.5.1 Cells
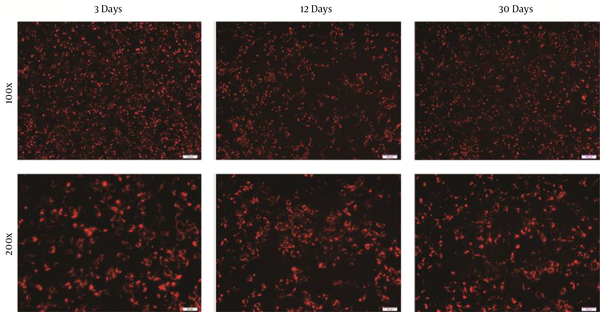
Huh7.5.1 cells were routinely maintained in a 10% FBS supplemented medium. It has been confirmed that Huh7 cells could be passaged and cultured over a long time in an ITS-supplemented medium, and a serum-free cultured system was established for later purification. Huh7.5.1 cells were sequentially passaged in lower FBS medium and ultimately into ITS-supplemented medium. After adaptation to the serum-free media, Huh7.5.1 cells were transfected with JFH-1 RNA. The infection rate of cells was about 47% after 3 days and sustained for over 1 month, as determined by the average number of HCV core-positive foci detected by immunofluorescence analysis (Figure 1).
Immunofluorescence staining of Huh7.5.1 cells fixed at 3, 12, and 30 days after transfection with JFH-1 ribonucleic acid in serum-free culture. Immunofluorescence assay was performed by incubating with anti-hepatitis C virus core (C7-50) (Red); (100×, scale bar: 100 µm; 200×, scale bar: 50 µm)
The HCV RNA level was highest at 30 days and remained stable after 3 and 12 days of culture (Table 1). The infectious titer was determined by immunofluorescence, showing that complete virus particles were produced. The titer of infectious HCV was highest at 3 days and remained stable between 12 and 30 days. Specific infectivity was calculated by dividing the infectious titer of the culture supernatants by the concentration of HCV RNA as previously reported (13). According to these calculations, the specific infectivity values of the culture medium were 3.695 × 10-2, 2.761 × 10-2, and 3.695 × 10-2 at each time point; however, the infectivity value at the 3rd day time point was relatively higher. The results indicated that Huh7.5.1 cells could persistently produce complete virus particles in serum-free media over 1 month.
| Time, d | Hepatitis C Virus Ribonucleic Acid, IU/mL | Infectious Titer, FFU/mL | Specific Infectivityb, FFU/RNA IU |
|---|---|---|---|
| 3 | 1.732 × 106 | 6.4 × 104 | 3.695 × 10-2 |
| 12 | 1.485 × 106 | 4.1 × 104 | 2.761 × 10-2 |
| 30 | 3.946 × 106 | 4.5 × 104 | 1.140 × 10-2 |
Infectivity of the Supernatant of JFH-1 Transfected Huh7.5.1 in Serum-Free Culturea
4.2. Purification of HCV Particles by Heparin-Affinity Chromatography and Magnetic Separation Technique
The culture medium with HCV particles was collected every 3 - 5 days after cell passage and concentrated approximately 50 folds using Centricon® centrifugal filter device with a 100 kDa cut-off. For the purification of HCV particles, two different methods were used. Since it was reported that HCV particles from infected human plasma could be purified by heparin-affinity chromatography, concentrated HCV particles were passed through a heparin-affinity column and eluted with increasing concentrations of NaCl.
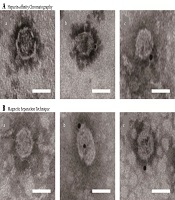
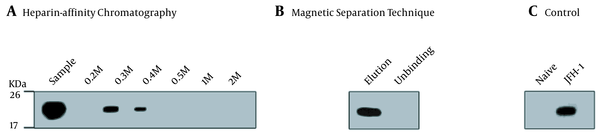
Most HCV particles detected by the western blot with anti-HCV E2 antibody AP33 were eluted with 0.3 M and 0.4 M NaCl (Figure 2A). Naïve and JFH-1 infected Huh7.5.1 cells were used as negative and positive controls for the western blot, respectively (Figure 2C). The diameter of the particles was within the range of 50 - 65 nm using electron microscopy (Figure 3A), suggesting that purification by heparin-affinity chromatography preserved the structure and morphology of viral particles.
Western blot analysis of purified JFH-1 viruses from: A, Heparin-Affinity chromatography; and B, Magnetic Separation technique. Lysates of naive Huh7.5.1 and JFH-1 transfected Huh7.5.1 were served as negative and positive controls, respectively; C, Blots were probed with anti-hepatitis C virus core (C7-50) and detected by HRP-labelled anti-mouse lgG and lgM. The positions of the molecular mass markers (KDa) are shown.
The magnetic separation technique was used to further purify HCV particles. A serum sample MM110 from an HCV-infected patient with high levels of anti-E2 antibody was biotinylated. It was used to capture the virus, which was then purified by SA-PMPs. The HCV particles were eluted with 0.1 M sodium citrate (pH = 3), separated, and purified (Figure 2B). The diameter of the particles was consistent (Figure 3B) with the size of the purified virus from heparin-affinity chromatography and a previously reported study (6).
Electron microscopy of purified JFH-1 viruses from: A, Heparin-Affinity chromatography; and B, Magnetic Separation technique. After purification, the eluted fraction was absorbed on microscopy grids and negative stained. The grids were observed by transmission electron microscopy (scale bar: 50 nm).
4.3. Detection of HCV Particles by Immune-Electron Microscopy
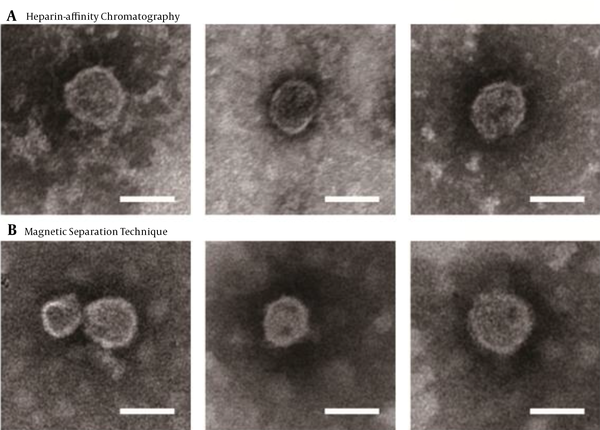
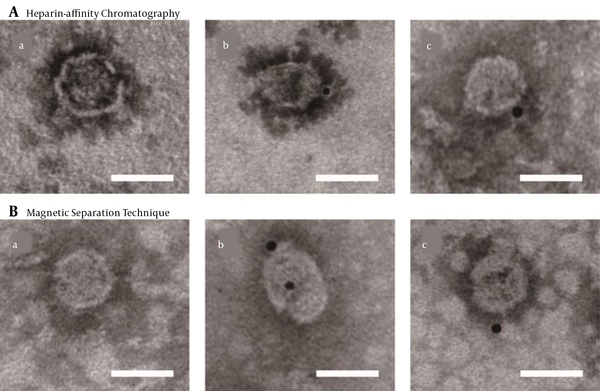
To further confirm the integrity of purified viral particles, immune-electron microscopy was performed using an HCV E2-specific antibody AP33. Bound antibodies were detected with Anti-Mouse IgG (whole molecule)-Gold (10 nm in diameter). The control grid was reacted with an unrelated primary antibody (Figure 4). The diameter of the structures binding to gold was within the range of 50 - 65 nm in size, indicating that viruses were complete after the purification with heparin-affinity chromatography and magnetic separation technique as visualized under immuno-electron microscopy (Figure 4).
Immuno-electron microscopy of purified JFH-1 virus from: A, Heparin-affinity chromatography; and B, Magnetic Separation technique. Purified viruses were absorbed on microscopy grids and incubated with anti-hepatitis C virus (HCV) envelope protein 2 (E2) antibody AP33. Bound antibodies were detected with Anti-Mouse IgG (whole molecule)-Gold (10 nm in diameter). The lowercase letter (a) indicated the grids with purified virus incubated with non-related primary antibody (an anti-brucellosis bp26 antibody), while (b) and (c) with anti-HCV E2 antibody (scale bar: 50 nm).
5. Discussion
It was previously reported that Huh7 cells could be cultured over a long period in a selenium-supplemented medium (13); nevertheless, a serum-free culture system had advantages for the simple purification and preparation of animal-origin-free virus particles. This study established a production system of HCV particles in the Huh7.5.1 cell line, which was adapted to serum-free media containing selenium. Under these conditions, the production rate of HCV was stable for 1 month (Figure 1), consistent with the situation in Huh7 (13). The excreted viruses in the medium were infectious and abundant (Table 1). The specific infectivity of serum-free cultured HCV was almost identical at each indicated time, revealing that this system was suitable for long-term culture and large-scale virus production.
The binding of HCV to heparin was used to effectively purify the viruses from the plasma of chronically infected patients previously (12). Therefore, this study developed heparin-affinity chromatography to purify HCV particles from serum-free cultures. It was suggested that ≥ 0.4 M NaCl was required to elute HCV in plasma (12). Nonetheless, in the current study, HCV particles in the culture supernatant were mainly eluted with 0.3 M and 0.4 M NaCl (Figure 2A), probably because the viruses were obtained by culture.
The visualization of HCV by electron microscopy showed no disruption of viral particles after heparin-affinity chromatography, and the concentration was high enough to be visualized (Figure 3A), with an average outer diameter of about 55 nm as previously reported (6). Furthermore, an electron micrograph of spherical structures was displayed by immunogold labeling. Purified viruses from both heparin-affinity chromatography and magnetic separation technique were specifically bound with gold (Figure 4A), indicating the presence of surface viral envelope glycoproteins.
The magnetic separation technique has become a trend for sample purification in biological analysis. The use of PEI beads was reported for the concentration of HCV particles (16) as a concentration method rather than purification because it lacks accurate selectivity. Anti-HCV E2 and other antibodies are produced when individuals are exposed to HCV and develop chronic infection (17). The serum from infected HCV patients containing such antibodies would effectively capture viral particles. Moreover, commercial streptavidin-conjugated magnetic beads are commonly used in this regard.
In this study, HCV was purified by streptavidin-conjugated magnetic beads bound to biotinylated anti-HCV antibodies from infected patients. This method was efficient and specific, as shown by the western blot (Figure 2B). Immuno-electron microscopy suggested that viral particles were complete, consistent with results obtained by heparin-affinity chromatography (Figures 3B and 4B).
The recovery percentage of heparin-affinity chromatography and magnetic separation technique was about 30% - 40% according to RT-qPCR and western blot assessments, indicating that heparin elution contained less than 50% of the input viral genomes (18). However, this study did not evaluate the infectivity of isolated HCV particles by the neutralizing assay; however, the infectious titer in Huh7.5.1 cells was measured, suggesting that the purified viruses had infectivity. Moreover, both methods should be run in parallel with the ultracentrifugation method, and the final products should be quantitatively analyzed.
The application of DDAs makes the HCV treatment more effective; nevertheless, the sustained antiviral response rate of treatment is higher than 90% (19). However, the therapeutic effects of different virus genotypes are different with high variation frequency of virus drug resistance and high price (20-23). Drug discovery and resistance studies on HCV become more and more important in the era of DAAs. The HCV replicon systems have become indispensable in the development of effective DAAs and the investigation of drug resistance (24), requiring a large number of infectious particles for high throughput anti-viral library assessments. These two new purification methods of HCV particles have shown potential application in this field.
In conclusion, this study established a production system of HCV particles from JFH-1 transfected Huh7.5.1 cells in a serum-free medium. The present study also developed two simple and efficient methods (i.e., heparin-affinity chromatography and magnetic separation technique), suitable for the large-scale purification of HCV particles.