1. Background
According to Globocan 2020, HCC as the sixth most frequent malignancy and one of the most lethal malignancies was the third leading cause of neoplasia-related death in 2020 worldwide (1). Although recent data provide evidence on the significant decrease of the HCC incidence and its mortality rates in high-risk regions, the incidence rates of HCC in so-called low‐risk regions (namely Europe, Northern America, Australia, South America) are either rising or appear to plateau at a higher level over the last few years (2). Accordingly, the identification and management of the modifiable major risk factors such as hepatitis B (HBV) or C virus (HCV) chronic infection, excessive alcohol consumption, aflatoxin, type 2 diabetes mellitus, obesity, and smoking are of essence to interrupt the upward dynamics of the HCC rates (1).
Chronic HCV infection has an annual incidence rate ranging from 3 to 5% per year (3). According to the WHO’s Global Hepatitis Report, about 71 million persons are infected with HCV worldwide (4). The carcinogenic potential of HCV has been extensively studied. According to previous studies, its pro-oncogenic effects on the infected cells are caused by both direct (i.e., DNA damage, oxidative mechanisms) and indirect (i.e., liver injury and fibrosis) mechanisms (5).
During two decades of interferon (IFN)-based therapy, obvious evidence from several studies has indicated that sustained virological response (SVR), obtained in < 50% of patients (6), reduced liver-related mortality and thereby the incidence rate of HCC (7-9). Data from the IFN era showed that achieving viral eradication (SVR) significantly reduced the risk of HCC, and this finding was a significant contribution to hepatologists (10, 11). However, the IFN-based therapy was ranked as suboptimal considering the low SVR rate (approximately 40 - 50%) and poor tolerance and limited access caused by strict criteria for the eligibility of patients (11).
The continuous research on the HCV viral genome has led to the discovery of direct-acting antivirals (DAAs). This finding was considered as one of the most significant advances in clinical medicine during the past decade and was associated with several benefits such as high efficacy (SVR rates > 95%), acceptable tolerability, short treatment duration (8 - 12 weeks), and simple administration (once-daily oral dosage) (12). Although the DAA therapy has been introduced recently, it has resulted in some benefits (e.g., improved liver function and portal hypertension and decreased liver fibrosis) in cirrhotic patients (12). According to the beneficial effects of SVR on the HCC occurrence reported in the IFN era, clinicians anticipated even further benefits, including a significant decrease in the HCC occurrence or recurrence, in cirrhotic patients treated with DAAs. However, in 2016, shadows were cast over such fantastic therapeutic triumph when two articles from Spain and Italy reported that the DAA therapy might favor the HCC occurrence or recurrence (13, 14). This issue was a hotbed of debate in more than 100 papers, letters, or communications; however, no conclusive result was achieved. While evidence suggests that the risk of HCC does not completely disappear after SVR, it is of great importance to determine the role of DAA therapy in hepatocarcinogenesis to show whether it is suppressing or promoting the development of HCC. As much data as possible on this topic is immediately required to better manage HCC surveillance, especially for cirrhotic patients.
2. Objectives
This study aimed to determine the long-term risk of de novo HCC and risk factors in patients with HCV genotype 1b compensated cirrhosis, following the achievement of SVR by DAA regimens. As a secondary objective, this study also aimed to assess the tumor aggressiveness and its impact on treatment decisions.
3. Methods
3.1. Patients
This multicentric cohort study analyzed the data from 479 consecutive patients with chronic HCV genotype 1b infection and compensated liver cirrhosis, treatment-experienced or naïve, treated with paritaprevir/ritonavir/ombitasvir, and dasabuvir (PrOD) +/- ribavirin (RBV) for 12 weeks in two tertiary centers in Northeastern Romania. The patients were prospectively followed up in The Institute of Gastroenterology Iasi, Romania, from November 2015 to December 2020. The patients were included in the study according to the Romanian National Health Insurance House’s criteria: (1) patients with HCV genotype 1b infection; and (2) compensated cirrhosis defined as F4 by transient elastography (≥ 13 kPa). We excluded patients aged below 18 years at the initiation of treatment and those with concomitant human immunodeficiency virus or hepatitis B virus infection, heavy alcohol intake, and documented malignant neoplastic disease, including HCC.
3.2. Monitoring During and After DAA Treatment
Clinical parameters were measured using standard laboratory techniques at the “St. Spiridon” Emergency Hospital’s laboratory. The laboratory tests (namely HCV RNA level, aspartate and alanine aminotransferases, bilirubin, alkaline phosphatase, gamaglutamyl transpeptidase, albumin, and international normalized ratio, serum creatinine, hemoglobin, platelet count, and alpha-fetoprotein) were performed before DAAs, at the end of treatment (EOT), three months after EOT (SVR), and whenever it was necessary. The diagnosis of HCV infection was decided based on the serum HCV RNA levels, measured with the COBAS TaqMan HCV quantitative test (Roche Molecular Systems, Inc. Branchburg, NJ) with a lower limit of quantification and detection of 15 IU/mL. The scores of Child–Pugh-Turcotte (CPT) and model of end-stage liver disease (MELD) were calculated at the baseline, end of treatment, and 12 weeks after the therapy.
We used transient elastography (FibroScan; Echosens, Paris, France) to perform the liver stiffness measurement (LSM) with 13 kPa as the cutoff point for cirrhosis (15).
3.3. HCC Surveillance
The HCC screening was performed at the baseline in all patients using abdominal ultrasonography (US), computed tomography (CT), or magnetic resonance imaging (MRI). US and serum a-fetoprotein (AFP) were performed every 3 - 6 months for all patients after the initiation of treatment.
3.4. Ethical Considerations
This study was approved by the National Ethics Committee, and written informed consent was obtained from all participants, while observing the principles of the Declaration of Helsinki.
3.5. Statistical Analysis
All data were statistically analyzed using SPSS software version 22.0 (IBM SPSS Inc., Chicago, IL, USA). The continuous variables were expressed as median (first-third quartiles) and compared using Student's t-test; however, the categorical variables were reported as frequencies and percentages and compared using chi-squared or Fisher's exact tests. The Kaplan-Meier method was used to calculate and plot the cumulative HCC incidence. Only complete data were analyzed in this study.
To compare the differences among the groups, the log-rank test was used. In this regard, a Cox proportional hazard model with a hazard ratio (HR) and 95% confidence interval (CI) generated by Cox regression was calculated in both univariate and multivariate analysis to detect the risk factors associated with the HCC occurrence. Moreover, we evaluated the relationship between AFP and the HCC occurrence using the area under the receiver operating characteristic curve (AUROC) analysis, and the optimal cutoff value was selected at the highest specificity and sensitivity from the receiver operating characteristic (ROC). Statistically, two-tailed P < 0.05 was set as the significance level in this study. Kolmogorov-Smirnov test was performed to check the normality of the data distributions.
4. Results
4.1. Participants’ Characteristics
Table 1 shows the demographic, clinical, biological, and imaging data of the study participants. The study included 479 patients treated with PrOD ± RBV, with a median age of 60 (52-73) years, who mainly encompassed female patients (54.5 %). Of the research participants, 32% had a history of antiviral treatment with IFN, and 16.5% received RBV associated with PrOD. The participants’ body mass index (BMI) was 27.76 ± 4.04 kg/m2. More than half of the patients (57.2%) had comorbidities, the most common ones of which were hypertension (36.5%) and type 2 diabetes mellitus (14%). All patients had compensated liver cirrhosis (according to CPT, 88.5% had a score of 5, and 11.5% had a score of 6).
| Parameters | Values a |
|---|---|
| Age (y), median (IQR) | 60 (52 - 73) |
| Gender, male/female | 218/261 (45.5/54.5) |
| BMI (kg/m2), mean ± SD | 27.76 ± 4.04 |
| Comorbidities | 274 (57.2) |
| Obesity | 16 (3.3) |
| Hypertension | 175 (36.5) |
| Diabetes mellitus | 67 (14) |
| Personal history of neoplasia | 16 (3.3) |
| IFN experienced | 153 (31.9) |
| Ribavirin | 79 (16.5) |
| Esofageal varices | 133 (27.8) |
| Small | 69 (51.8) |
| Medium | 21 (15.7) |
| Large | 43 (32.3) |
| Child-Pugh score | |
| 5 | 424 (88.5) |
| 6 | 55 (11.5) |
Abbreviations: BMI, body mass index; IFN, interferon.
a Values are expressed as No. (%) unless otherwise expressed.
4.2. Direct-Acting Antiviral Therapy Outcomes
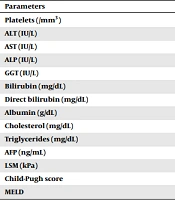
All patients completed the 12-week treatment course and achieved SVR after the DAA therapy. The mean follow-up period was 60.11 ± 3.87 months. A statistically significant decrease was recorded at the ALT, AST, GGT, and AFP levels and LSM (P < 0.001) in SVR compared to baseline. Table 2 represents further information in this regard.
| Parameters | Baseline | SVR | P-Value |
|---|---|---|---|
| Platelets (/mm3) | 138 (101 - 181) | 146 (105 - 193) | < 0.001 |
| ALT (IU/L) | 87 (60 - 123) | 24 (19 - 33) | < 0.001 |
| AST (IU/L) | 83 (56 - 128) | 22 (19 - 30) | 0.001 |
| ALP (IU/L) | 94 (76 - 116) | 92 (73 - 137) | < 0.001 |
| GGT (IU/L) | 69 (43 - 110.5) | 32 (22 - 46) | < 0.001 |
| Bilirubin (mg/dL) | 0.92 (0.68 - 1.27) | 0.74 (0.42 - 1.05) | 0.233 |
| Direct bilirubin (mg/dL) | 0.43 (0.33 - 0.59) | 0.43 (0.28 - 0.7) | < 0.001 |
| Albumin (g/dL) | 4.04 (3.73 - 4.38) | 4.29 (3.94 - 4.6) | 0.943 |
| Cholesterol (mg/dL) | 160 (146 - 192) | 183 (152 - 210.25) | 0.039 |
| Triglycerides (mg/dL) | 107 (83 - 125) | 99.5 (77 - 125.75) | 0.545 |
| AFP (ng/mL) | 9.28 (5.2 - 16.92) | 3.89 (2.76 - 6.75) | 0.002 |
| LSM (kPa) | 21.55 (16.78 - 32.63) | 10.6 (7.1 - 15.3) | < 0.001 |
| Child-Pugh score | 5 | 5 | 0.235 |
| MELD | 8.1 (7 - 10) | 7.5 (7 - 8) | 0.321 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; AFP, alpha-fetoprotein; LSM, liver stiffness measurements; MELD, model for end-stage liver disease.
a Values are expressed as median value and range.
b Wilcoxon signed-rank test was used to compare values at baseline and SVR.
c P < 0.05 was considered to indicate a statistically significant difference.
4.3. Incidence and Risk Factors of HCC Occurrence
After the mean follow-up of 60.11 ± 3.87 months, 23 patients (4.8%) developed HCC. The 1-, 3-, and 5-year cumulative incidence rates of HCC were 1.1, 1.9, and 2.6%, respectively. The mean period from the beginning of the treatment to the HCC diagnosis was 19.6 ± 13.7 months.
All patients in our cohort study had compensated liver cirrhosis (Child-Pugh A 5 or 6). Baseline factors contributing to the HCC occurrence following DAAs were then assessed (Table 3). The patients who were diagnosed with HCC (n = 23) were older (63 vs 59 years, P = 0.022) and more likely to be male (57 vs 55%, P = 0.448) compared to the non-HCC patients (n = 456).
| Variables | HCC = 23 | No HCC = 456 | P-Value |
|---|---|---|---|
| Age | 63.04 ± 10.1 | 59 ± 8.14 | 0.022 |
| Gender, male/female, (male%) | 13/10 (57) | 251/205 (55) | 0.448 |
| PLT, × 104/μL | 12.6 ± 50.4 | 14.8 ± 63.9 | 0.109 |
| BMI | 26.9 ± 3.7 | 27.7 ± 4.04 | 0.156 |
| AFP baseline | 15.8 ± 23.5 | 13.9 ± 9.7 | 0.696 |
| ActiTest | 0.76 ± 0.13 | 0.69 ± 0.18 | 0.037 |
| ALT, U/L | 112.78 ± 69.07 | 103.54 ± 71.56 | 0.545 |
| AST, U/L | 110.87 ± 51.68 | 101.25 ± 66.88 | 0.497 |
| GGT, U/L | 89.68 ± 76.42 | 93.98 ± 81.67 | 0.809 |
| ALP, U/L | 113.95 ± 40.73 | 98.95 ± 33.91 | 0.046 |
| Treatment experienced, Yes (%) | 205 (45) | 13 (56.5) | 0.312 |
Abbreviations: PLT, platelets; BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; AFP, alpha-fetoprotein; ALP, alkaline phosphatase.
a Values are expressed as mean ± SD unless otherwise indicated.
Furthermore, higher AST and AFP levels, ActiTest scores, and lower PLT counts were observed at baseline in the HCC patients than the non-HCC patients. Table 4 presents the variables detected by multivariate Cox hazard regression analysis, which are significantly associated with the HCC development. According to the AUROC analysis for the HCC development regarding the AFP level recorded in all patients, the best cutoff value for the AFP level assessed at DAAs cessation was 10 ng/mL. After adjusting the intervening variables, we found an HR of the HCC development with a serum AFP level at EOT > 10 ng/mL of 3.01 (95% CI, 1.092 - 8.340, P = 0.046). A serum AFP level at EOT > 10 ng/mL was an independent risk factor of the HCC occurrence.
| Variables | Multivariate Analysis | ||
|---|---|---|---|
| HR | 95% CI | P | |
| Male (gender) | 1.25 | 0.866 - 1.825 | 0.278 |
| Age > 60 years | 2.08 | 1.409 - 3.066 | 0.002 |
| Obesity | 1.32 | 0.182 - 9.577 | 0.792 |
| Treatment experienced | 1.53 | 0.981 - 2.404 | 0.094 |
| AFP EOT > 10 ng/mL | 3.01 | 1.092 - 8.340 | 0.046 |
Abbreviations: AFP, alpha-fetoprotein; EOT, end of treatment.
4.4. HCC Features and Management
Of the patients who developed HCC, 12 patients (52.2%) met the Milan criteria, and according to the BCLC classification, a majority of them were in class A (39.1%) (Table 5). Regarding the imaging characteristics of the tumor, most patients had a single nodule (65.2%), and the presence of the capsule was highlighted in most cases (82.6%). Malignant portal vein thrombosis (PVT) was demonstrated in 26% of the patients, and local/distant metastases was noticed in 13% of the patients.
| Variables | HCC Cases (n=23) |
|---|---|
| Milan | |
| Within | 12 (52.2) |
| Beyond | 11 (47.8) |
| BCLC | |
| A | 9 (39.1) |
| B | 4 (17.4) |
| C | 7 (30.4) |
| D | 3 (13) |
| Imaging feature | |
| Single tumor | 15 (65.2) |
| Capsule visualization | 19 (82.6) |
| Metastasis | 3 (13) |
| Malignant PVT | 6 (26) |
Abbreviations: BCLC, Barcelona clinic liver cancer; PVT, portal vein thrombosis.
a Values are expressed as No. (%).
The therapeutic management of the patients with HCC was performed according to the BCLC classification. Most patients (n = 11, 47.8%) received curative treatment by surgical resection, among whom histopathological examination detected a moderately differentiated tumor (G2) in five patients, a poorly differentiated tumor (G3) in five patients, and a well-differentiated tumor (G1) only in one patient. Three patients (27%) had HCC recurrence after a mean of 6 ± 5.2 months and were subsequently addressed for systemic therapy.
5. Discussion
The high rate of SVR, along with an excellent tolerability profile and extended indication for antiviral treatment at all stages of chronic HCV infection, enhanced expectations for lower HCC rates after viral eradication with the DAA therapy among clinicians worldwide. Such enthusiasm was overshadowed after the publication of data indicating, in contrast to expectations, unexpectedly higher novo and recurrent HCC after DAA treatment in patients with chronic HCV infection (13, 14). These findings have led to the emergence of an "avalanche" of studies assessing this risk (Table 6).
| References | Research Population | HCC Incidence |
|---|---|---|
| Retrospective studies | ||
| Conti et al. (14) | 285 cirrhotic patients treated with DAAs | 3.16% |
| Singer et al. (16) | 30183 DAA-treated, 12948 IFN-treated, and 137502 untreated | 1.18/100 PY |
| Nahon et al. (7) | 336 DAA-treated, 495 IFN-treated with SVR, and 439 IFN-treated without SVR | 2.6/100 PY |
| Ioannou et al. (17) | 21948 DAA-treated, 35871 IFN-treated, 4535 DAA + IFN treated | 1.32/100 PY |
| Janjua et al. (10) | 8871 IFN-treated and 3905 DAA-treated | 6.9/1000 PY |
| Kanwal et al. (18) | 22500 DAA-treated | 1.18/100 PY |
| Prospective studies | ||
| Cheung et al. (19) | 406 DAA-treated and 261 untreated | 4% |
| Mettke et al. (20) | 148 DAA-treated and 184 untreated | 2.90/100 PY |
| Carrat et al. (21) | 7344 DAA-treated and 2551 untreated | 1.40/100 PY |
| Poordad et al. (22) | 2211 DAA-treated | 1.4% |
| Sangiovanni et al. (23) | 1285 DAA-treated (n = 1285) | 3.1/100 PY |
Abbreviations: DAAs, direct-acting antivirals; IFN, interferon; SVR, sustained virological response; PY, person-year.
Contrary to our expectations, we observed the sustained HCC incidence rates for up to 5.1 follow-up years in HCV-free cirrhotic patients after the DAA treatment. The cumulative 5-year risk was 2.6%, and this value exceeded the cutoff beyond which HCC surveillance was cost-effective (24). Nonetheless, similar to the findings of other studies, the findings of the present study support the incidence of HCC, with no evidence on the high occurrence of de novo HCC after the DAA therapy. For example, a prospective study by Cheung et al. (19) revealed no increased risk of HCC during or 12 months after the DAA cessation in 406 patients with HCV-related decompensated cirrhosis. Furthermore, the researchers concluded that the HCC incidence of 4.2% observed during the first six months after DAAs was identical to the incidence rate in the untreated control group. Similarly, a cohort study on 22,500 DAA treated patients reported a significant decrease in the HCC risk in patients who achieved SVR compared to the non-responders (0.90 vs. 3.45 cases of HCC/100 person-years) (18). Interestingly, the same authors in their later article on DAA-treated patients, who were followed up over 3.5 years after SVR, reported the 1-, 2-, and 3-year cumulative risks of HCC to be 1.1, 1.9, and 2.8%, respectively (24).
In contrast to the IFN era, the increased efficacy and tolerability of DAA allowed treatment in patients with more risk factors for HCC than patients in historical cohorts treated with IFN, the most relevant of which were older age, diabetes mellitus, and liver cirrhosis. Regarding the presence of risk factors, the existence of inhomogeneity in the DAA and IFN groups may explain the higher incidence rates of HCC in patients treated with the new antivirals. Recent data have indicated that the risk factors associated with de novo and recurrent HCC after DAA treatment are represented by older age, advanced liver fibrosis, and the absence of SVR (14, 16, 17). In the present study, age > 65 years at baseline and a cutoff value of AFP at EOT = 10 ng/mL were independent risk factors associated with the HCC occurrence.
Although there are abundant data regarding the HCC occurrence and recurrence after DAAs, few reports assessed the behavior of HCC and access to curative therapy after the DAA treatment (25-31). Regarding tumor aggression, the patients with HCC in the present study revealed aggression less frequently than those reported in other studies. For example, a recent study by Fayoume et al. demonstrated that the frequency of cases with multiple HCC and infiltrative HCC was significantly higher among the DAA-treated patients than in naïve patients with HCC (28). In contrast, most HCC patients in the present study had a single nodule (65.2%). The portal vein invasion was observed in 26% of patients, and there were local and distant metastases in 13% of the patients. Furthermore, according to the BCLC classification, most patients were in classes O and A; thus, they had access to curative treatment. Similarly, Fatima et al. reported that, considering the BCLC stages, multiplicity, malignant PVT, and local spread through malignant lymphadenopathy, the HCC pattern did not differ between patients treated with IFN and those treated with DAAs (29).
The main limitation of the present study was the presence of no control group of untreated patients to compare the characteristics and the prognosis of HCC after DAAs. Other limitations were the lack of patients with decompensated cirrhosis and the use of a single DAA regimen in our cohort.
5.1. Conclusions
The present study revealed no evidence of the high HCC occurrence after long-term follow-up of patients with HCV genotype 1b infection and liver cirrhosis, who achieved SVR following the DAA treatment. However, the cumulative 5-year risk remained above the cutoff point, above which the HCC screening becomes cost-effective. According to these findings, clinicians should maintain HCC surveillance in those with liver cirrhosis at the time of SVR. The tumor phenotype does not seem to be more aggressive after DAAs, and the access to curative therapy is similar to that of the HCC associated with other liver diseases. The evaluation of the HCC pattern requires prospective case-control studies comparing the clinical-biological and imaging markers of tumor aggression between the HCC cases after DAAs and naïve patients.