1. Background
Chronic hepatitis C virus (HCV) may either remain silent for a long time or progress enough to cause advanced liver fibrosis, hepatocellular carcinoma (HCC), and even liver failure (1). Liver fibrosis may become evident in patients after a long period of remaining asymptomatic. Severe liver fibrosis is an important problem that may cause liver failure.
Evaluation of liver fibrosis secondary to chronic HCV infection has a considerable role in determining the prognosis and clinical course of the infection. Liver fibrosis has been suggested as a strong predictor of complications and mortality (2, 3). Although liver biopsy is still the gold standard procedure for evaluating fibrosis, it is an invasive method with some complications such as bleeding, pain, gallbladder rupture, and shock. Sampling errors, as well as interobserver and/or intraobserver variability, and the inhomogeneous nature of fibrosis are other limitations that may affect the optimal evaluation of liver biopsy samples (4). In addition, liver biopsy is contraindicated in patients with severe Cirrhosisand tendency to bleed. For these reasons, non-invasive indicators and techniques for estimating fibrosis, such as serum biomarkers and imaging methods, have been studied and suggested to determine the stage of fibrosis due to chronic HCV infection (5, 6). Particularly, serum biomarkers have gained attention because their measurement does not require highly qualified personnel or expensive kits.
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Pin1) modulates cytokine production via activating T cells and eosinophils and also shows apoptotic functions. The suppression of Pin1 reduces the production of transforming growth factor (TGF)-β1 and granulocyte-macrophage colony-stimulating factor (GM-CSF), modulating inflammation (7-9). It has been shown that Pin1 promotes liver fibrosis via inducing the production of monocyte chemoattractant protein (MCP)-1 and tumor necrosis factor (TNF α), as two important inflammatory cytokines (10).
2. Objectives
We aimed to assess the association between the serum levels of Pin1, as a non-invasive serum biomarker, and liver fibrosis in patients with chronic HCV infection. The serum level of Pin1 was compared between healthy controls and patients with chronic HCV infection to ascertain its possible relationship with liver fibrosis and its stage.
3. Methods
3.1. Participants
In this cross-sectional study, all patients with HCV infection (genotype 1b) were consecutively enrolled. The patients were over 18 years old and had yet received no treatment, for whom liver biopsy was performed in the Gastroenterology Department, Faculty of Medicine, Gazi University. Age- and sex-matched healthy volunteers (the recruitment ratio of 2:1) were also included in the study as controls.
Inclusion criteria for the study were positivity for anti-HCV and HCV RNA and liver biopsies compatible with HCV infection. Quantitative real-time PCR was used to determine HCV RNA load in serum samples using Qiagen Artus HCV RG RT-PCR kit (Qiagen, Hilden, Germany) (sensitivity: 34 IU/mL) and a Rotor-Gene 6000 (Corbett Research, Qiagen, Hilden, Germany) device in accordance with the manufacturer’s recommendations. Genotype was also determined by real-time PCR using the Gene Q (Qiagen, Hilden, Germany) automated device. All the patients had genotype 1b at the time of diagnosis. Since most patients with chronic HCV in our country carry genotype 1b, only these patients were included in the study to eliminate the effects of different genotypes on fibrosis and Pin1 serum levels.
The presence of another genotype other than 1b, positivity for hepatitis B surface antigen, HIV positivity, chronic intake of alcohol more than 20 mg per day, histopathological evidence of autoimmune or metabolic liver disease, severe cardiac or renal diseases, any malignancy, infectious diseases, and use of immunosuppressive or hepatotoxic drugs were regarded as exclusion criteria. The healthy volunteers included in the control group did not have any liver diseases, and their liver enzymes and abdominal ultrasound results were normal. Also, healthy subjects were clinically asymptomatic with no evidence for infections or carcinoma.
3.2. Histopathological Evaluation
Liver biopsies were performed percutaneously by the same skilled gastroenterologist using disposable Menghini needles (Hepafix 16 gauge; Braun Melsungen AG, Melsungen, Germany) in a sterile manner. The biopsy samples containing hepatic tissues with a minimum diameter of 20 - 25 mm and more than 12 complete portal triads were fixed in 10% formalin buffer and stained with hematoxylin & eosin and Masson trichrome. All liver biopsy samples were evaluated by the same pathologist who was skilled in hepatopathology and blinded to the data. Liver necroinflammatory activity (NIA) and liver fibrosis were evaluated according to the Ishak Scoring System classification (11). Liver fibrosis was scored from F0 to F6: F0; without any fibrosis, F1; fibrous expansion in a few portal areas regardless of fibrous septa, F2; fibrous expansion in numerous portal areas regardless of fibrous septa, F3; fibrous expansion of most portal areas with rare portal to portal bridging, F4; fibrous expansion with marked bridging (portal to portal and portal to central), F5; significant bridging with a few nodules, and F6; the presence of cirrhosis (11). Accordingly, fibrotic scores of < 3 and ≥ 3 were classified as mild and advanced fibrosis, respectively.
3.3. Clinical and Laboratory Data Acquisition
Clinical and demographic information were retrospectively collected and recorded by a physician blinded to the patients’ data to avoid bias. The anthropometric measures of weight and height were recorded, and body mass index (BMI) was calculated by dividing the weight by the height in meters squared and expressed as kg/m2. Before performing liver biopsy, venous blood samples were obtained from the antecubital veins of the patients and healthy controls between 8: 30 and 10:00 a.m., after fasting for at least 12 hours. A serum separator tube (SST) was used to allow blood to clot for 30 minutes. Then the samples were centrifuged at 1000 g for 15 minutes to separate serum samples, which were immediately stored at -80°C until analysis. We avoided repeated freeze-thaw cycles to prevent obtaining different results. Complete blood cell count (CBC), including hemoglobin and white blood cell, platelet, neutrophil, and lymphocyte counts, was performed using a Beckman Coulter Gen-S automated analyzer (High Wycombe, UK) within the first two hours of blood sampling. Fasting blood glucose level and lipid parameters were determined using commercial Elisa kits (Roche Cobas Integra 800, Indianapolis, Ind., USA). Low-density lipoprotein (LDL) cholesterol level was evaluated using Friedewald’s equation (12). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), albumin, and γ-glutamyl aminotransferase (GGT) levels were measured by a Roche Modular System auto-analyzer (Roche Cobas Integra 800, Indianapolis, Ind., USA).
3.4. Measuring Serum Pin1 Level
A commercial enzyme-linked immunosorbent assay (ELISA) kit was used in full compliance with the manufacturer’s instructions to measure serum Pin1 level (human protein (peptidylprolyl cis/trans isomerase) NIMA-interacting 1 (Pin1) ELISA kit, Catalogue number: CSB-E11308h, Wuhan, Hubei, China). The procedure was based on the quantitative sandwich enzyme immunoassay technique. The antibodies of Pin1 were pre-coated in the microplate, and the kit offered a high sensitivity (< 0.39 pg/mL) and specificity. The optical density of each well was determined at 450 nm and a time interval of five minutes using an automatic ELISA reader with a reference filter wavelength of 540 nm. The exact detection range of the ELISA kit was between 1.56 and 100 pg/mL. Intra- and inter-assay variance coefficients were regarded as 8% and 10%, respectively. For measuring the optical density of standards, regression-correlation analysis was applied using serum samples with known Pin1 levels.
3.5. Statistical Analysis
Statistical analysis was performed using SPSS software version 23 (SPSS Inc., Chicago, IL, USA) and MedCalc version 12 (Mariakerke, Belgium). The variables were investigated visually (histograms, probability plots) and via analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk test) to determine whether or not they were normally distributed. The descriptive statistics of mean and standard deviation (SD) were used to present normally distributed continuous variables. Non-normally distributed and ordinal variables were represented by median and interquartile range (IQR). As Pin1 serum levels were not normally distributed, non-parametric methods were conducted to compare this and other ordinal variables between the study groups. The Mann-Whitney U test was performed to compare serum Pin1 levels and non-normally distributed biochemical factors, and the student t-test was used for the comparative analysis of normally distributed variables. The Spearman correlation coefficient was used to estimate the correlation between NIA, fibrosis stage, and serum Pin1 level. The efficiency of serum Pin1 level for predicting advanced fibrosis in patients with chronic HCV was analyzed using Receiver Operating Characteristics (ROC) curve analysis. Regarding ROCs, Youden Index analysis was performed to determine the best cut-off value with the highest sensitivity and specificity. When evaluating the area under the curve, a 5% type-1 error level was used to accept a statistically significant predictive value of test variables. A P value less than 0.05 was considered statistically significant for all analyses.
3.6. Ethical Statement
This study was in full compliance with the ethical guidelines of the Helsinki Declaration and was approved by the Institutional Review Board of Faculty of Medicine, Gazi University. All the participants read and signed an informed consent form before beginning the study.
4. Results
Ninety-four consecutive biopsy samples were obtained from patients with an established diagnosis of chronic HCV (genotype 1b) infection. Also, 47 healthy eligible (based on the inclusion and exclusion criteria) volunteers were included in the study. The patients and healthy individuals were matched for characteristics such as age and gender. Among the patients and healthy controls, 55.3% of the participants were female, with a median age of 52 years. Median interquartile range (IQR) of serum Pin1 level was significantly higher in the HCV infected group compared to healthy volunteers [33.94 (21.15) pg/mL vs. 26.82 (8.85) pg/mL, respectively, P = 0.007]. Clinical and laboratory data of healthy volunteers and HCV (genotype 1b) infected patients have been summarized in Table 1.
| Factors | Healthy Volunteers (n = 47) | HCV-Infected (Genotype 1b) (n = 94) | P-Value |
|---|---|---|---|
| Age, y | 52 (21 - 75) | 52 (22 - 75) | 0.68 |
| Females/n | 26/47 | 52/94 | 0.99 |
| BMI, kg/m2 | 25.21 4.18 | 26.56 (6.10) | 0.52 |
| Serum Pin1, pg/mL | 26.82 (8.85) | 33.94 (21.15) | 0.007 |
| Hemoglobin, g/dL | 13.80 (1.18) | 13.79 (3.0) | 0.22 |
| Platelet, /mm3 × 103 | 260.40 (90.9) | 185.10 (107.7) | < 0.001 |
| WBC, /mm3 | 7260 (4220) | 5760 (2050) | 0.11 |
| Albumin, mg/dL | 4.20 (0.30) | 4.30 (0.88) | 0.94 |
| Fasting plasma glucose, mg/dL | 98 (18) | 97 (21) | 0.25 |
| Triglycerides, mg/dL b | 147.77 ± 79.52 | 124.43 ± 69.87 | 0.16 |
| Total cholesterol, mg/dL | 201 (36) | 164 (48) | 0.65 |
| LDL, mg/dL | 127 (37) | 89.80 (45) | 0.01 |
| VLDL, mg/dL b | 28.72 ± 16.53 | 24.52 ± 11.80 | 0.65 |
| HDL, mg/dL | 47 (18) | 46 (16) | 0.54 |
| ALT, IU/mL | 20 (8) | 25 (40) | 0.005 |
| AST, IU/mL | 18 (8) | 27 (30) | < 0.001 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; WBC, white blood cell count.
aNon-normally distributed variables were expressed via median and interquartile range (IQR).
b Normally distributed as mean ± SD (standard deviation).
Regarding liver histopathological evaluation, mild fibrosis (fibrosis score < 3) was observed in 77 (82%) patients while 17 (18%) revealed advanced fibrosis (a score ≥ 3). Overall, 27 (28.7%) patients showed the fibrosis score of 0, and 27 (28.7%), 23 (24.4%), 9 (9.5%), 5 (5.3%), and 3 (3.1%) presented the fibrosis scores of 1, 2, 3, 4, and 5, respectively.
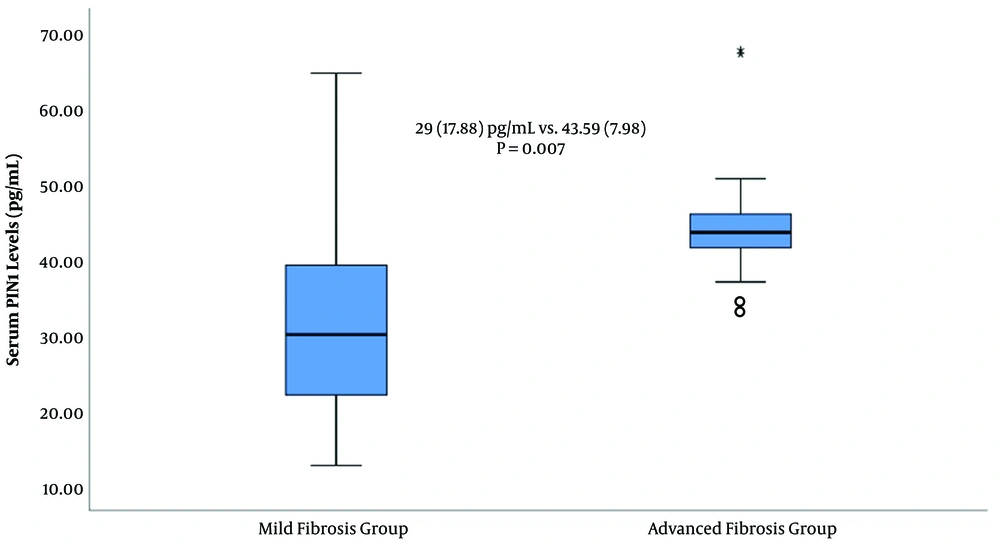
The median age of patients with mild and advanced fibrosis was comparable [52 (21 - 75) vs. 53.5 (42 - 73) years, respectively, P = 0.49]. Gender was also comparable between the two groups (P = 0.19). In addition, the median IQR of histopathological NIA score was significantly lower in the mild compared with the advanced fibrosis group [4 (3) vs. 8 (4), respectively, P < 0.001]. Likewise, HCV RNA (IU/mL) levels showed no statistically significant difference between the mild and advanced fibrosis groups (P = 0.54). However, the median value of serum Pin1 level showed a statistically significant difference between the mild and advanced fibrosis groups [29 (17.88) vs. 43.59 (7.98) pg/mL, respectively, P = 0.007, Figure 1].
The clinical and laboratory findings of patients with mild and advanced liver fibrosis due to HCV (genotype 1b) infection have been represented in Table 2.
| Factors | Mild Fibrosis (n = 77) | Advanced Fibrosis (n = 17) | P-Value |
|---|---|---|---|
| Age, y | 52 (21 - 75) | 53.5 (42 - 73) | 0.49 |
| Females/n | 45/77 | 7/17 | 0.19 |
| BMI, kg/m2 | 26.56 (5.78) | 26.94 (4.82) | 0.70 |
| Serum Pin1, pg/mL | 29 (17.88) | 43.59 (7.98) | < 0.001 |
| Hemoglobin, g/dL | 13.95 (2.43) | 11.60 (2.66) | 0.02 |
| Platelet, /mm3 × 103 | 193.30 (97.30) | 120.0 (96.50) | 0.005 |
| WBC, /mm3 | 6200 (2155) | 4005 (2600) | < 0.001 |
| Albumin, mg/dL | 4.20 (0.85) | 4.30 (1.32) | 0.27 |
| Fasting plasma glucose, mg/dL b | 104.33 ± 32.73 | 97.71 ± 14.61 | 0.55 |
| Triglycerides, mg/dL | 128.89 (73.46) | 105 (48.81) | 0.71 |
| Total cholesterol, mg/dL | 172.23 (44.83) | 157.71 (35.38) | 0.44 |
| LDL, mg/dL | 98.79 (41.71) | 85.14 (27.40) | 0.43 |
| VLDL, mg/dL | 25.39 (12.51) | 20.74 (7.22) | 0.23 |
| HDL, mg/dL b | 48.25 ± 21.18 | 51.29 ± 35.33 | 0.74 |
| ALT, IU/mL b | 42.15 ± 58.88 | 57.93 ± 50.23 | 0.07 |
| AST, IU/mL b | 40.20 ± 35.52 | 65.50 ± 55.17 | 0.011 |
| NIA | 4 (3) | 8 (4) | < 0.001 |
| HCV RNA, IU/mL | 1.5 × 106 (103-5.6 × 106) | 1.9 × 106 (103-6.1 × 106) | 0.54 |
Abbrevitions: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HCV, hepatitis C virus; HDL, high-density lipoprotein; LDL, low- density lipoprotein; NIA, necro-inflammatory activity; WBC, white blood cell count.
aNon-normally distributed variables were expressed via median and interquartile range (IQR).
b Normally distributed variables as mean ± SD (standard deviation).
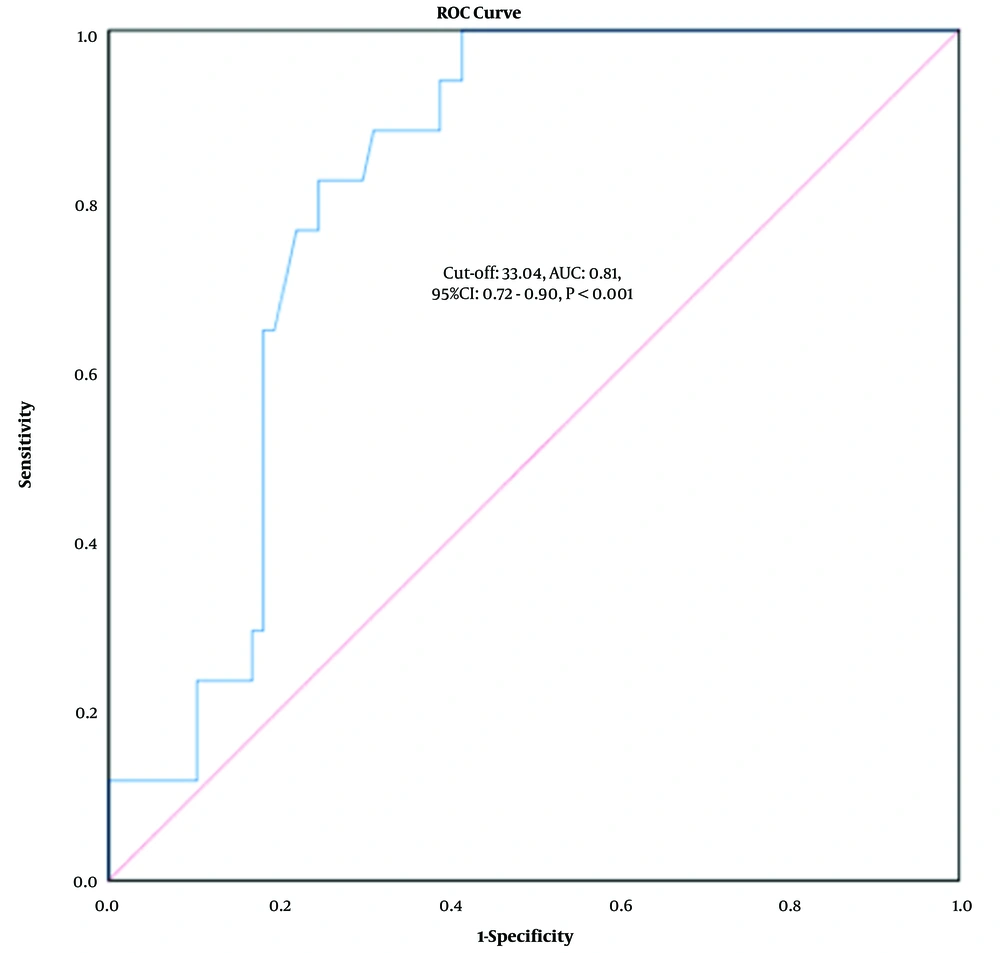
According to ROC analysis, the cut-off value of 33.04 had the highest sensitivity (100%) and specificity (68.4%) for distinguishing advanced from mild fibrosis, with an AUC of 0.81 (95% CI: 0.72 - 0.90, P < 0.001, Figure 2).
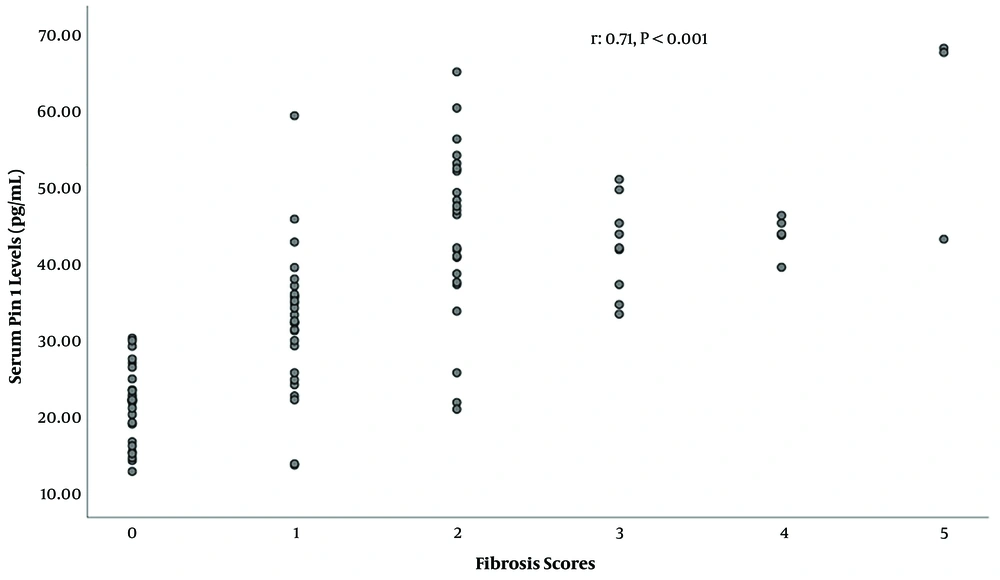
In HCV infected patients, serum Pin1 level showed a significant positive correlation with liver fibrosis score (r = 0.71, P < 0.001, Figure 3) and histopathological NIA score (r = 0.42, P < 0.001).
5. Discussion
As the main finding of this study, we noticed a statistically significant difference in serum Pin1 levels between the patients infected with chronic HCV (genotype 1b) and healthy volunteers. Also, serum Pin1 level seemed to be a good predictor of advanced liver fibrosis in HCV infected patients. Serum Pin1 levels strongly correlated with liver fibrosis and its progression, and ROC analysis demonstrated that serum Pin1 level was associated with advanced fibrosis in patients with HCV (genotype 1b) infection. To our knowledge, this is the first study reporting a relationship between serum Pin1 level and liver fibrosis stage in HCV-infected patients.
Advanced liver fibrosis may lead to poor prognosis and serious complications such as decompensated cirrhosis, HCC, and even liver failure. Esophageal varices and HCC are more likely to be detected in patients with advanced liver fibrosis due to HCV infection (13-15). In this study, we concluded that serum Pin1 levels significantly correlated with advanced liver fibrosis, which is compatible with the literature (13-15).
Lim et al. (16) emphasized that the life cycle of HCV generally depended on various cellular factors, and based on screening a small selected RNA library, they proved that Pin1 was involved in HCV progression. They also demonstrated that a decrease in Pin1 expression correlated with a decline in HCV-infected/producing hepatocytes and HCV replication in HCV (HCVcc) infected cells grown in culture media. Another valuable finding of their work was that they detected an increase in HCV replication as Pin1 level increased. They concluded that Pin1 modulated HCV spread and contributed to HCV-induced liver damage (16). Our study supported the results of Lim et al., indicating that Pin1 levels were effective in distinguishing HCV patients from healthy individuals, and the fact that serum levels of Pin1 were associated with liver fibrosis. Therefore, Pin1 may potentially be utilized as a non-invasive serum marker to predict and grade liver fibrosis severity in the patients infected with HCV. Our observation regarding the association between serum Pin1 level and liver inflammation and fibrosis supported the results obtained in molecular studies on HCV pathophysiology.
Pin1 is overexpressed in many human malignancies and in more than 50% of HCCs. The overexpression of Pin1 in a non-transformed human hepatic cell line was shown to change hepatocytes’ morphology, functionality, and life span. In addition, Pin1 suppression reduced HCC tumorigenesis (17). In another study, the suppression of Pin1 and cytokine production was followed by a decline in tumor growth. Pin1 also inhibited apoptosis in HCC tumor cells through modulating the anti-apoptotic function of survivin (7, 13). As liver fibrosis progresses, the possibility of HCC development increases. Our findings are in line with the molecular and cellular studies suggesting a role for Pin1 in HCC pathogenesis. The results of our study showed that serum Pin1 levels positively correlated with liver fibrosis.
It has been suggested that Pin1 has a critical role in the development of non-alcoholic steatohepatitis (NASH), and the suppression of this molecule was found as a new therapeutic modality in a rodent model. In NASH patients, Pin1 was also associated with fibrosis. It has been shown that TGF-β, connective tissue growth factor, and the profibrogenic cytokines promoting fibrosis significantly decreased in Pin1-deficient mice, completely repressing fibrosis markers (10, 18). Our study confirmed an association between serum Pin1 level and liver inflammation and fibrosis in patients with HCV (genotype 1b) infection.
As concluded in our study, serum Pin1 level positively correlated with liver fibrosis severity. This observation may be explained by the fact that Pin1 participates in regulating the expression of the proteins involved in fibrosis. . Determining liver fibrosis indirectly based on serum Pin1 levels in HCV-infected patients may open a novel path for designing new therapeutic approaches to suppress Pin1 and improve the clinical course of HCV patients with advanced fibrosis.
The results of our study are consistent with those of previously published in vivo and in vitro studies indicating that Pin1 increases inflammatory cytokines’ expression and release from bone marrow-derived macrophages (19). It has been reported that Pin1 inhibits gluconeogenesis and enhances the metabolic actions of insulin in a variety of inflammatory disorders (19-21). The statistically significant positive correlation observed between the NIA score and serum Pin1 level in this study suggested that inflammation would be suppressed and disease progression would be slower among HCV infected patients with low serum Pin1 levels.
Our study has several limitations. It is noteworthy that Pin1 regulates peroxisome proliferator-activated receptor gamma (PPAR γ) activity, which itself is a reflection of serum adiponectin level. Adiponectin has been used as a biomarker for screening the efficacy of PPAR γ treatment (22, 23). Accordingly, it is suggested to assess serum adiponectin level and investigate its association with Pin1 levels in future studies to elaborate our results. Also, studies involving more patients should be conducted to better evaluate this issue. Nevertheless, we tried to overcome these limitations by including the patients infected only with HCV genotype 1b and conducting the experiments in the same center and by the same hepato-pathologist. The lack of consecutive sampling over time for some of the patients to monitor the fluctuations of serum Pin1 levels during and after treatment was another limitation of our study. So, conducting a multi-center study to record serum Pin1 fluctuations at the pre-and post-treatment phases would give additional information on this topic. Human procollagen III propeptide, collagen-IV, hyaluronic acid (HA), and laminin (LN) are well-established and cost-effective markers to evaluate liver fibrosis. Therefore, these tests can also be beneficial to confirm our observations.
5.1. Conclusions
In conclusion, serum Pin 1 level can be used as a non-invasive predictor of advanced liver fibrosis in patients with chronic HCV (genotype 1b) infection.