1. Background
Non-alcoholic fatty liver disease (NAFLD) has affected about 24 - 30% of the world's population (1, 2). In the last decades, the prevalence of overweight and obesity has increased exponentially, resulting in an increase in the prevalence of NAFLD, which comprises a range of diseases from simple steatosis to non-alcoholic steatohepatitis (NASH). Based on the natural history of disease, NASH may progress to cirrhosis and hepatocellular carcinoma (3).
Obesity, physical inactivity, and diets rich in saturated fat, simple carbohydrates, and fructose are the most prominent causes of NAFLD (4, 5). Dyslipidemia, insulin resistance, hypertension, and type 2 diabetes are the clinical manifestations of NAFLD. NAFLD occurs when the synthesis and uptake of fatty acids by the liver exceeds the rate of catabolism and export by very-low-density lipoprotein (VLDL), characterized by the accumulation of lipids within hepatocytes, mainly due to insulin resistance, high carbohydrate consumption, and de novo lipogenesis (6).
Animal models that represent the hepatic histopathology and pathophysiology of human NAFLD/NASH have been useful tools for investigating the mechanisms involved in the etiology of NAFLD and developing new drugs (5). Kanuri and Bergheim found that six weeks of a diet rich in fructose and fat led to hepatic steatosis, besides significant changes in inflammatory factors. Moreover, a combined diet containing both fat and fructose, compared to a high-fat or fructose-rich diet, is the most effective factor in NAFLD progression to more advanced stages (5).
Carbon tetrachloride (CCl4) has been used for many years to induce liver damage and fibrosis in animal models. When combined with a Western diet rich in fat and fructose, it can induce oxidative stress, inflammation, and apoptosis. As fibrosis progresses in hepatocytes, fatty liver leads to steatosis; CCl4 induces NAFLD through this mechanism (7-9). In previous studies, steatosis and hepatic fibrosis were induced via CCl4 injection at doses of 0.2 mL/kg (8, 10), 0.5 - 0.7 mL/kg (11, 12), 1 mL/kg (13), and 2 mL/kg (14). Due to variable doses and durations of CCl4 injection for the induction of NAFLD, besides the possibility of hepatotoxicity rather than NAFLD induction, a method based on diet is needed to develop an optimal model.
2. Objectives
This study aimed to propose an animal model of NAFLD due to the consumption of a high-fat diet and CCl4 injection and to modify previously introduced models.
3. Methods
3.1. Animals and Diets
A total of 48 male Wistar rats (202 ± 15 g), obtained from Pasteur Institute of Iran (Tehran, Iran), were divided into six groups (n = 8) and kept in floor pens with wood shavings and hay and straw as environmental enrichment. The animals had ad libitum access to feed and water and were kept in a 12: 12 h light/dark cycle at 20 - 24°C. The group characteristics and dietary compositions are presented in Table 1. The intervention was performed for six weeks to induce hepatic steatosis. In this regard, Zhang et al. (2020) examined the effect of CCl4 injection on NAFLD induction; the CCl4 dose in the present study was based on the results of their study. Besides, because we did not achieve definite results in the groups during the pilot study, we modified the prescribed dose of CCl4 to an intermediate level (0.1 mL/kg) between the two doses used by Zhang et al. (0.2 and 0.08 mL/kg) (9).
The rats were fed a chow diet; the experimental design of the intervention is shown in Table 1. The standard diet for the animals contained 4.30 kcal per gram with 3.87% fat (soy oil), 17.46% casein protein, 68.7% carbohydrates, 8.97% minerals, and 1% vitamins. The food intake measurements, CCl4 injection, and gavage of olive oil and fructose 55% (Zar Grain Refinery, Alborz, Iran) were performed at the same time daily. The rats were monitored weekly by weighing. Based on the ethical principles for laboratory animal use, sampling was performed from the rat tails in the second and fourth weeks and at the end of the intervention (sixth week before anesthesia and dissection); blood samples were taken from the abdominal aorta.
All animals received humane care according to the Principles of Laboratory Animal Care, formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals designed by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86–23, revised in 1985).
3.2. Biochemical Analysis
The rats were anesthetized by an intraperitoneal injection (IP) of ketamine (50 mg/kg) and xylazine (5 mg/kg) (Merck, Germany). Next, blood samples were collected and centrifuged at 3000 rpm for ten minutes to prepare the sera. The levels of urea, creatinine, alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT), as well as lipid profile including triglyceride (TG), total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C), and high-density lipoprotein-cholesterol (HDL-C), were measured by specific enzymatic kits (Pars Azmoon, Tehran, Iran) and BT 3500 Autoanalyzer (Medsystem, USA).
3.3. Liver Histology
At the end of the sixth week, the rats were sacrificed for pathophysiological evaluations. After collecting blood samples, livers were instantly removed and washed with physiological saline. After collecting the liver tissue samples via incisions, they were kept in 10% buffered formaldehyde and embedded in paraffin for hematoxylin-eosin (H&E) staining and NAFLD grading. The NAFLD Activity Score (NAS), including hepatocyte ballooning, lobular inflammation, and steatosis, was determined to examine the NAFLD grade.
3.4. Statistical Analysis
Data are expressed as median (interquartile range). Significant differences between the groups were determined by Kruskal-Wallis H test. Also, Mann-Whitney U test was used to compare the groups. P-values less than 0.05 were considered statistically significant.
3.5. Ethics Approval
This study was carried out according to the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health (NIH Publication No. 85–23, revised in 1996). The study protocol was authorized by the local ethics committee (IR.GOUMS.REC.1400.124). We attempted to minimize animal suffering and reduce the number of animals used.
4. Results
4.1. Weight and Food Intake
The average weight of the rats is shown in Figure 1. The analysis of the groups’ weight data from week 2 to week 6 showed that the interventions did not cause a significant difference in weight compared to the control group. Also, there was no significant difference between the intervention groups. The average food intake is presented in Figure 2. The results of the analysis of food intake showed that in the second week of the intervention, the food intake of the FF group increased compared to the control group (P = 0.028). In the fourth week, the average food intake significantly decreased in the FFC1 (P = 0.001) and FC1 (P = 0.025) groups. Also, in the sixth week of the intervention, the food intake decreased in the FFC1 (P = 0.006) and FC1 (P = 0.016) groups, whereas the C1 (P = 0.002) and C2 (P = 0.043) groups showed a significant increase in food intake.
Average of Food intake of groups in 2nd, 4th and 6th week [*P < 0.05; **P < 0.001 (significance of intervention groups vs control); FFC1, fructose, fat, CCl4 (1: 4 in olive oil); C1, CCl4 (1: 4 in olive oil); C2, CCl4 (1: 6 in olive oil); FF, fructose, fat; FC1, fructose, CCl4 (1: 4 in olive oil)].
4.2. Liver Enzymes
The results of data analysis in the second week of the intervention showed significant changes in the serum levels of liver enzymes between the NAFLD model groups. The ALT (P = 0.003) and ALP (P = 0.000) levels in the FC1 group showed considerable growth compared to the control group. Among the NAFLD groups, the level of AST in the FFC1 (P = 0.039), C1 (P = 0.025), and C2 (P = 0.001) groups and the level of GGT in the FFC1, C1, C2, and FF groups significantly increased compared to the control group in the second week of the intervention (P ≤ 0.001).
In the fourth week of the intervention, the AST level in the C2 and FC1 groups (P = 0.000), the ALP level in the FF group (P = 0.001), and the GGT level in the FFC1, C1, and C2 groups (P = 0.000) increased significantly. Conversely, the ALP level in the C2 group (P = 0.000) and the GGT level in the FF (P = 0.004) and FC1 (P = 0.017) groups significantly decreased. After six weeks of the intervention, the ALT and ALP levels showed decreasing trends in the FC1 group (P = 0.000) compared to the control group. On the other hand, the ALT level in the C1 group (P = 0.000), the AST level in the FFC1 and C1 groups (P = 0.000), and the GGT level in the C1 (P = 0.020), FF (P = 0.000), and FC1 (P = 0.000) groups increased compared to the control group (Table 2).
| Parameters | Week 2 | Week 4 | Week 6 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | FF | FC1 | FFC1 | C1 | C2 | FF | FC1 | FFC1 | C1 | C2 | FF | FC1 | FFC1 | |
| ALT | 36.30 | 43.90 | 88.90 | 122.6 a | 36.30 | 57.40 | 52.60 | 88.94 | 76.10 | 57.40 | 131.63 b | 60.75 | 48.40 | 20.40 b | 142.66 b |
| AST | 203.8 a | 102.80 b | 208.50 | 215.00 | 228.80 a | 205.80 | 338.40 b | 173.20 | 106.30 b | 195.60 | 275.00 b | 141.70 | 161.64 | 211.30 | 283.00 b |
| ALP | 203.00 | 345.30 | 401.60 | 683.00 b | 212.00 | 370.00 | 221.00 b | 520.40 b | 454.00 | 395.00 | 374.00 | 303.40 | 410.60 | 121.00 b | 383.00 |
| GGT | 2.94 b | 2.36 b | 3.05 b | 1.55 | 3.50 b | 3.70 b | 5.30 b | 0.20 a | 0.30 a | 3.50 b | 1.74 a | 1.15 | 5.70 b | 10.40 b | 1.10 |
Mean Values of Liver Enzymes of Groups 2nd, 4th and 6th Week
4.3. Lipid Profile
The lipid profile data indicated that in the second week of the intervention, the level of TG in the C1 (P = 0.002) and FC1 (P = 0.001) groups, TC in the C2 group (P = 0.017), LDL in the FC1 group (P = 0.000), and HDL in all groups (P ≤ 0.05) increased significantly compared to the control group. Based on the results, in the fourth week of the intervention, TG in the C2 (P = 0.005) and FF (P = 0.000) groups, TC in the C2 group (P = 0.000), LDL in the FC1 group (P = 0.001), and HDL in all groups (P = 0.000) significantly increased compared to the control group.
In the sixth week of the intervention, changes in TG showed a decreasing trend in the C2 group (P = 0.001). On the other hand, TG in the FF and FC1 groups (P = 0.000), TC in the C1 (P = 0.002), FF (P = 0.042), and FC1 (P = 0.000) groups, LDL in the FC1 group (P = 0.000), and HDL in all groups, except FC1 (P = 0.000), significantly increased compared to the control group. Based on the findings, the level of LDL significantly decreased in the FFC1 and C1 groups compared to the control group (P = 0.000); however, in the FC1 group, these changes showed an increasing trend (P = 0.001) (Table 3).
| Parameters | Week 2 | Week 4 | Week 6 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | FF | FC1 | FFC1 | C1 | C2 | FF | FC1 | FFC1 | C1 | C2 | FF | FC1 | FFC1 | |
| TG | 62.40 a | 24.50 | 27.80 | 67.00 b | 42.00 | 34.00 | 56.00 a | 69.00 b | 48.00 | 47.00 | 47.00 | 21.00 b | 58.2 b | 136.0 b | 35.00 |
| TC | 65.00 | 90.00 a | 41.00 | 64.50 | 72.00 | 60.50 | 97.00 b | 67.00 | 65.00 | 65.00 | 82.00 a | 68.00 | 76.50 a | 95.00 b | 58.00 |
| LDL | 8.70 | 8.10 | 10.80 | 13.30 b | 8.70 | 9.50 | 8.20 | 9.30 | 12.20 b | 9.80 | 8.90 b | 7.30 | 11.00 | 14.10 b | 10.00 b |
| HDL | 44.10 a | 60.20 a | 31.50 a | 33.90 a | 47.70 a | 37.00 b | 55.00 b | 49.80 b | 44.50 b | 39.10 b | 61.00 b | 46.80 b | 34.90 b | 17.30 | 19.80 b |
Mean Values of Lipid Profiles of Groups 2nd, 4th and 6th Week
4.4. Renal Indicators
The serum urea and creatinine levels were also measured to assess renal activity and possible damage. The results in the second week of the intervention indicated that in the C2 group, the serum levels of urea and creatinine significantly decreased compared to the control group (P ≤ 0.001). In the fourth week of the intervention, the urea level significantly decreased in all groups, except C2 (P ≤ 0.05); however, no significant changes were observed in the creatinine level. The results in the sixth week of the intervention showed that the urea levels decreased in all groups (P ≤ 0.050); however, no significant change was observed in the creatinine levels.
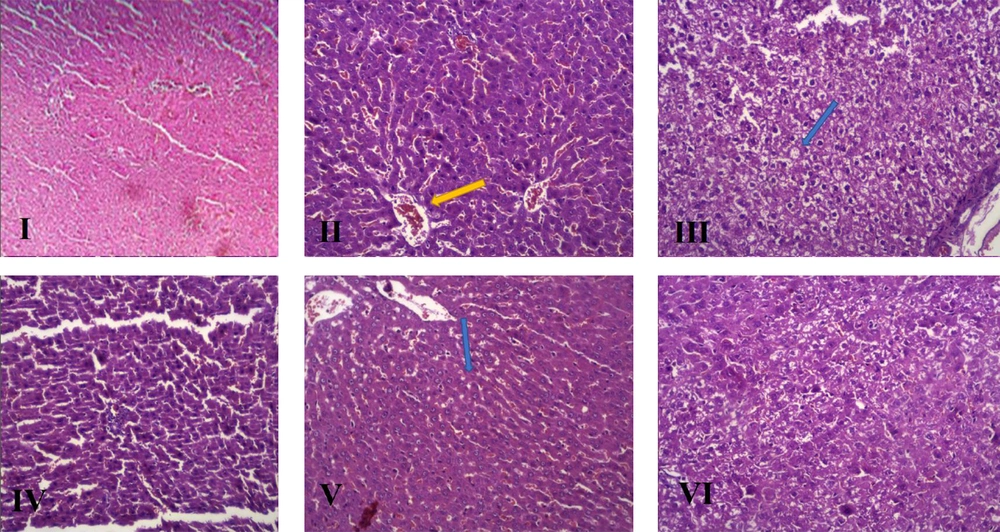
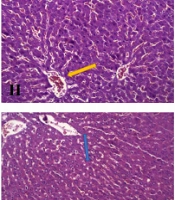
4.5. Hepatic Histological Analysis
The histopathological features and changes in the groups after H&E staining of the liver are presented in Table 4 and Figure 3, and the NAS index classification criteria was mentioned in Table 5, respectively.
| Histopathological Changes | FFC1 | C1 | C2 | FF | FC1 |
|---|---|---|---|---|---|
| Hepatocyte ballooning | 1 | 2 | 1 | 2 | 0 |
| Sinusoidal congestion | - | + | + | - | + |
| Sinusoidal dilation | - | + | + | - | + |
| Portal vein congestion | + | + | + | + | + |
| Portal vein dilation | + | + | + | - | + |
| Central vein congestion | + | + | + | + | + |
| Bile duct proliferation | - | - | + | - | + |
| Portal vein inflammation | + | + | + | - | + |
| Lobular inflammation | 1 | 1 | 0 | 0 | 1 |
| Kupffer cell hyperplasia | + | + | + | + | + |
| Hepatocyte polymorphism | + | + | - | - | + |
| Prominent nucleolus | + | + | + | + | + |
| Hepatocytes with eosinophilic cytoplasm | - | + | - | - | + |
| Focal necrosis | - | + | - | - | + |
| Steatosis (%) | 1 | 3 | 0 | 1 | 3 |
| #NAS | 3 | 6 | 1 | 3 | 4 |
Histopathological Changes in the Study Groups in the Sixth Week of the Intervention
| #NAS | Activity |
|---|---|
| 0 | No activity |
| 1 - 2 | Mild activity |
| 3 - 5 | Moderate activity |
| 6 - 8 | Marked activity |
Score of NAFLD Index Classification Criteria
5. Discussion
Animal models play an essential role in clarifying the pathophysiological mechanism of NAFLD. Models that mimic the histopathology and pathophysiology of every stage of human NAFLD can provide a better understanding of its pathogenesis and progression. Nevertheless, there are no accurate animal models representing the complete disease spectrum in a suitable timeframe, because the indices of NAFLD vary from animals to animals.
In this regard, Jensen et al. claimed that one of the reasons for the non-reproducibility and insignificance of comparisons in preclinical studies of NAFLD can be possible changes in sampling and non-standard sample sites (15-17). The present study proposed a model of NAFLD in Wistar rats by using CCL4, olive oil, and fructose for six weeks. We also studied differences in body weight, food intake, liver function tests, and serum biochemical parameters between the groups and within the groups in three time intervals.
Considering the changes in the serum levels of biochemical parameters (e.g., increased ALT and AST as serum markers of liver damage), as well as changes observed in the H&E staining of liver tissues, induction of NAFLD can be confirmed in the intervention group compared to the healthy controls. Also, since the gold standard method for the diagnosis and evaluation of NAFLD is pathology, by measuring hepatocyte indices, such as hepatocyte ballooning, lobular inflammation, and steatosis, besides calculating the NAS, NAFLD induction can be ensured. Generally, an optimal model has a higher level of steatosis and NAS.
Unlike previous studies which added fructose to drinking water (with ad libitum access of the animals), fructose in the present study was administered at a specific amount by intragastric gavage to eliminate the bias of excess calorie (18). The present results showed that treatment with 0.1 mL/kg of 25% (v/v) CCl4 solution in olive oil, along with 20% fructose/day for six weeks, resulted in simple steatosis, moderate activity of NAFLD, and a significant increase in the serum levels of TG, TC, LDL, and GGT. Recent studies have suggested that in male Wistar rats, consumption of drinking water with 20% fructose for six weeks led to the development of macrovesicular hepatic steatosis, increased body weight, and increased serum TG levels, without increasing TC or transaminase levels (18, 19). Another study showed that 20% fructose for 16 weeks resulted in microvesicular steatosis in Wistar rats and increased their body weight, without increasing the serum TG and transaminase levels (20). It is worth mentioning that 20% fructose alone, if administered ad libitum, cannot produce the NAFLD phenotype.
CCl4 is one of the chemical factors, commonly used to induce NAFLD. It is also considered to be an extremely toxic chemical agent (21). Nevertheless, the use of CCl4 alone induces fibrosis, not obesity or insulin resistance. Therefore, it is not an ideal model of NAFLD, and it is often combined with another diet when modeling NAFLD (15, 22). CCl4 is usually dissolved in vegetable oils and intraperitoneally injected into rats at a dose of 0.2 - 2 mL/kg over 6 - 12 weeks (23). CCl4, by producing activated oxygen-free radicals, destroys the hepatocellular structure and function. The peritoneal injection and intragastric administration of CCl4 every two weeks can induce extensive liver damage and increase the levels of transaminase and TG (24, 25) Although these models can easily and quickly cause fatty liver, this type of treatment increases the risk of mortality due to poisoning. Besides, the pathogenesis, histomorphological changes, and progression of disease in models are different from those of the human fatty liver (22).
In the present study, to reduce CCl4 toxicity, we first developed an experimental model of NAFLD by using CCl4 at a dose of 0.1 mL/kg, but in two proportions of 25% (C1) and 16.66% (C2), dissolved in olive oil; we compared the results with a control diet. In the sixth week of the intervention, the increase in body weight and food intake was similar in the C1 and C2 groups as compared to the control group. An increase in liver enzymes and TC was also observed following the intraperitoneal injection of CCl4 in the C1 group. Besides, treatment with 0.1 mL/kg of 25% (v/v) CCl4 solution in olive oil resulted in the marked activity of NAFLD, steatosis, and inflammation, while steatosis was not observed with 16.66% (v/v) CCl4.
CCl4, by producing activated oxygen-free radicals and covalent binding of these metabolites to the cell components, increases the synthesis of lipids and decreases their transport from hepatocytes, because it significantly inhibits the secretion of VLDL- and HDL-associated triglycerides and cholesterol esters (26); this can result in steatosis or fatty liver (27). The present results indicated that 0.1 mL/kg of 25% (v/v) CCl4 dissolved in olive oil could be used in combination with other diets for six weeks to induce NAFLD in Wistar rats.
Finally, to induce NAFLD, we used the following models: 12.5% fructose in combination with 12.5% olive oil (FF); 0.1 mL/kg of 25% (v/v) CCl4 dissolved in olive oil in combination with 45% fructose and 35% olive oil (FFC1); and 0.1 ml/kg of 25% (v/v) CCl4 solution in olive oil in combination with 20% fructose (FC1). In the sixth week of the intervention, changes in body weight, food intake, liver enzymes, and lipid profile were similar in the FF and FFC1 groups compared to the control group. Mild steatosis was observed in the FF and FFC1 groups. On the other hand, in the FC1 group, the rats showed significant differences in body weight and food intake compared to the control and C1 groups. The decrease in food intake in the FC1 group compared to the control group was due to increased calorie intake from fructose consumption; these results are consistent with previous studies (19).
Moreover, the FC1 group showed a significant increase in the serum levels of TG, TC, LDL-C, and GGT, while the FF group showed a significant decrease in HDL-C compared to the FC1 and FF groups. The increase in serum lipids in the FC1 group can be related to the ability of fructose to stimulate de novo lipogenesis, because fructose may directly stimulate lipogenic transcriptional factors, including sterol regulatory element-binding protein 1 (SREBP1c) and carbohydrate response element binding protein (ChREBP) in the liver, activating every stage of de novo lipogenesis (28). In the FC1 group, steatosis was also found by the accumulation of fat inside hepatocytes and inflammatory cells, but no ballooning degeneration occurred in the liver; this finding is related to the fact that fructose inhibits hepatic fatty acid β-oxidation, which causes fat accumulation in the liver (29).
Moreover, fructose increases the level of free fatty acids by stimulating the activity of enzymes in the de novo lipogenesis pathway, thereby increasing the synthesis and esterification of TG in the liver (30). Transaminases are used mainly to assess biochemical changes in the liver; an increase in these enzymes is an indicator of inflammatory response to injury (31). In this study, there was no increase in transaminases in the FC group. We hypothesized that treatment for more than six weeks may result in the greater inflammation of liver cells and elevation of transaminases. However, we found an increase in the GGT level of the FC1 group, which was in line with the histological finding of bile duct proliferation.
5.1. Conclusion
The present results showed that six weeks of a low-dose CCl4 combined with 20% fructose led to the development of steatosis in male Wistar rats. However, CCl4 alone, increased hepatic steatosis and caused dyslipidemia as the main criteria for NAFLD. Therefore, this model can be the most effective experimental method for drug testing and clarifying the pathophysiology of NAFLD.
![Average of the weight of groups in 2nd, 4th and 6th week [FFC1, fructose, fat, CCl<sub>4</sub> (1: 4 in olive oil); C1, CCl<sub>4</sub> (1: 4 in olive oil); C2, CCl<sub>4</sub> (1: 6 in olive oil); FF, fructose, fat; FC1, fructose, CCl<sub>4</sub> (1: 4 in olive oil)]. Average of the weight of groups in 2nd, 4th and 6th week [FFC1, fructose, fat, CCl<sub>4</sub> (1: 4 in olive oil); C1, CCl<sub>4</sub> (1: 4 in olive oil); C2, CCl<sub>4</sub> (1: 6 in olive oil); FF, fructose, fat; FC1, fructose, CCl<sub>4</sub> (1: 4 in olive oil)].](https://services.brieflands.com/cdn/serve/3170b/1e915ca8a002bd2b35bf4d8b5fd60a21132a56aa/hepatmon-117696-i001-F1-preview.webp)
![Average of Food intake of groups in 2nd, 4th and 6th week [*P < 0.05; **P < 0.001 (significance of intervention groups vs control); FFC1, fructose, fat, CCl<sub>4</sub> (1: 4 in olive oil); C1, CCl<sub>4</sub> (1: 4 in olive oil); C2, CCl<sub>4</sub> (1: 6 in olive oil); FF, fructose, fat; FC1, fructose, CCl<sub>4</sub> (1: 4 in olive oil)]. Average of Food intake of groups in 2nd, 4th and 6th week [*P < 0.05; **P < 0.001 (significance of intervention groups vs control); FFC1, fructose, fat, CCl<sub>4</sub> (1: 4 in olive oil); C1, CCl<sub>4</sub> (1: 4 in olive oil); C2, CCl<sub>4</sub> (1: 6 in olive oil); FF, fructose, fat; FC1, fructose, CCl<sub>4</sub> (1: 4 in olive oil)].](https://services.brieflands.com/cdn/serve/3170b/114db86e514ada8ab0f2a2efee8d370d71b08dea/hepatmon-117696-i002-F2-preview.webp)