1. Background
Hepatocellular carcinoma (HCC) is the second most common pediatric liver tumor and accounts for 30% of hepatic malignancies in this age group and 0.5% of all childhood malignancies (1). Overall, HCC in the pediatric age group is rare and much less common than adults (2). HCC is more common after the age of 10 and is the most common hepatic malignancy in adolescents (3, 4). Most of the HCCs diagnosed before the age of 10 are secondary to an underlying disease such as hepatitis B related cirrhosis and metabolic diseases like tyrosinemia (5). In this study we have investigated cases of hepatocellular carcinoma in the pediatric age group (under 18 years of age) during the last 10 years in our institution to find out the clinicopathologic characteristics of hepatocellular carcinoma in the pediatric age group in the largest referral center of pediatric hepatobiliary surgery in the South of Iran.
2. Methods
In the last 10 years (2005 - 2016), all of the cases of HCC in the pediatric age group (< 18 years of age) have been extracted from the archives of the pathology department of the affiliated hospitals of Shiraz University of Medical Sciences. During these years we found 30 cases of HCCs in the patients of the pediatric age group removed in these years. We also investigated the clinical chart of the patients to find out more about the clinicopathologic features of the disease.
3. Results
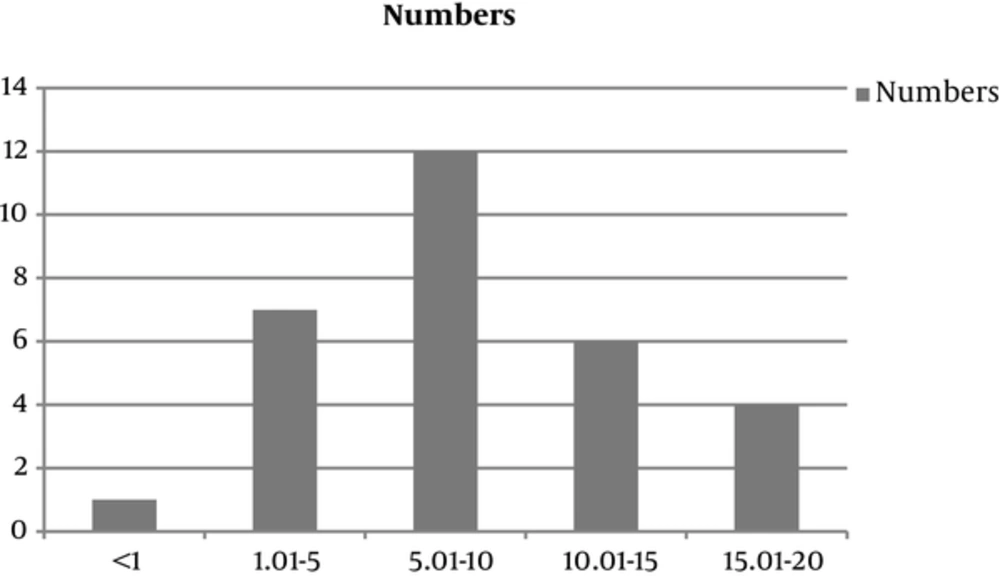
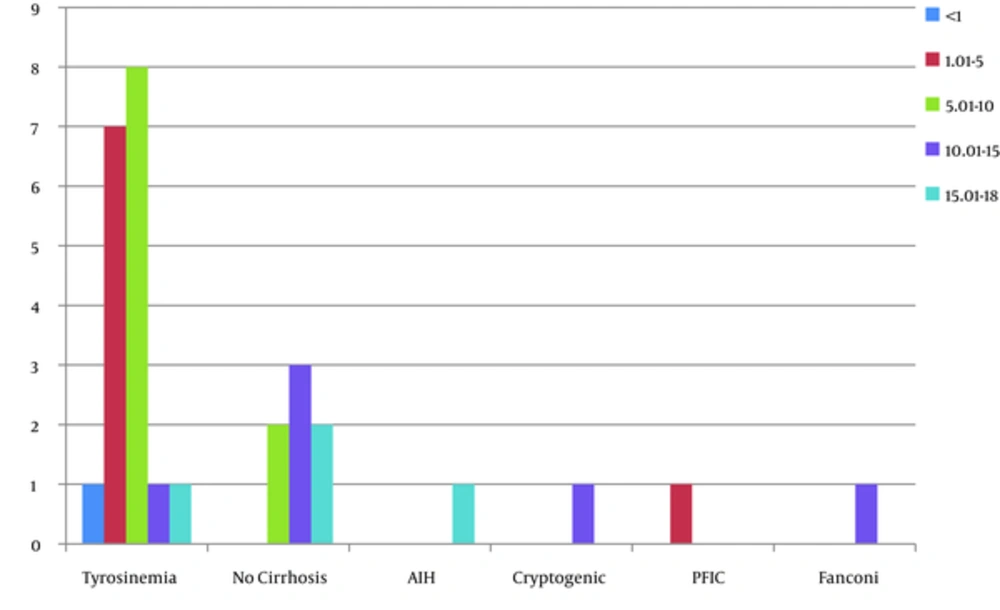
During the last 10 years, we found 30 cases of HCC removed in the patients below the age of 18. Among these 33 cases, there were 16 boys and 14 girls (M/F = 1.14/1). The youngest patient was 9 months old and the oldest, 18 years (Mean = 8.99 ± 5.2). Twenty patients (66%) were affected from liver cirrhosis. Fifteen (50%) cases showed multiple nodules of HCC, 14 (46.6%) cases have been located in the left lobe of the liver, and 10 (33.3%) cases in right. Three patients were presented with the fibrolamellar variant, all the others have been well differentiated HCC, and no case of moderate to poorly differentiated HCC has been referred to our hospital. The most common underlying cause of cirrhosis in 18 (60%) cases has been tyrosinemia. There have been 7 patients with no underlying cirrhosis and in this pediatric age group only 1 of the 23 cirrhotic patients was cryptogenic with no known underlying cause of cirrhosis. Nineteen patients have been transplanted. The largest HCC has been 17.7 cm and the mean tumor size was 5 ± 5.1 cm (range 0.5 - 20 cm). Most of our patients (n = 27/30, 90%) had increased levels of AFP, i.e. higher than 5 IU/L with the range of 620 ± 1048.7 IU/L. Among the patients, 21 patients are alive and completely well, most of which have been transplanted i.e. 16 (84.2%) patients out of 19 transplanted patients are well and alive, however, only 5 (45.45%) out of 11 nontransplanted patients are alive. Table 1 shows the details of clinicopathologic findings of the patients.
| No. | Age, y | Sex | Location | Number | Size, cm (Largest Diameter) | Underlying Disease | Cirrhosis | AFP(IU/L) Nl = 5 IU/L | Liver Transplant | Follow Up | Histologic Subtype |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | Male | Diffuse | Multiple | 2.5 | Tyrosinemia | + | 12 | + | Alive | Well diff HCC |
| 2 | 10 | Male | Right lobe | 1 | 2 | Tyrosinemia | + | 251.6 | - | Died | Well diff HCC |
| 3 | 17 | Male | Right lobe | 1 | 2 | Autoimmune hepatitis | - | 10 | + | Alive 2 years post Tx | Well diff HCC |
| 4 | 14 | Female | Left lobe | Multiple | 4 | Tyrosinemia | + | 51.2 | + | Alive 4 years post Tx | Well diff HCC |
| 5 | 15 | Female | Right lobe | 1 | 12 | - | - | 15 | - | Died | Fibrolamellar HCC |
| 6 | 3 | Male | Left lobe | 1 | 1 | PFIC | + | 8.3 | + | Alive, 2 years post Tx | Well diff HCC |
| 7 | 5 | Female | Left lobe | Multiple | 8 | Tyrosinemia | + | 400 | + | Alive 4 years post Tx | Well diff HCC |
| 8 | 4 | Female | Right lobe | 1 | 1 | Tyrosinemia | + | 553 | + | Alive 3 years post Tx | Well diff HCC |
| 9 | 5 | Female | Right lobe | 1 | 3 | Tyrosinemia | + | 4000 | + | Alive 1 year post Tx | Well diff HCC |
| 10 | 17 | Male | Diffuse | Multiple | 8 | Tyrosinemia | + | 1000 | - | Died | Well diff HCC |
| 11 | 5 | Male | Left lobe | 2 | 0.5 | Tyrosinemia | + | 1.9 | + | Alive, 2 years post Tx | Well diff HCC |
| 12 | 15 | Male | Left lobe | 1 | 2 | Unknown | + | 1000 | + | Died | Well diff HCC |
| 13 | < 1 | Male | Right lobe | 3 | 1.7 | Tyrosinemia | + | 12 | + | Alive,2.5 years post Tx | Well diff HCC |
| 14 | 10 | Female | Left lobe | 1 | 8 | - | - | 13 | - | Alive | Well diff HCC |
| 15 | 1 | Female | right lobe | 2 | 1.2 | Tyrosinemia | + | 50 | + | Died, 15 month post Tx | Well diff HCC |
| 16 | 11 | Male | Both lobes and hilum | 2 | 12.5 | - | - | 45 | + | Died 7 months post Tx | Fibrolamellar HCC |
| 17 | 8 | Male | Both lobes | Multiple | Allagile Sx | + | 1650 | - | Alive | Well diff HCC | |
| 18 | 2 | Male | Left lobe | 1 | 2 | Tyrosinemia | + | 619 | + | Alive | Well diff HCC |
| 19 | 1 | Male | Left lobe | 1 | 1 | Tyrosinemia | + | 751 | + | Alive, 1 year post Tx | Well diff HCC |
| 20 | 11 | Male | Both lobes | Multiple | 3.7 | - | + | 267 | - | Died | Well diff HCC |
| 21 | 18 | Female | Left lobe | 1 | 20 | - | - | 1.4 | + | Alive, 3 years post Tx | Well differentiated HCC |
| 22 | 10 | Female | Left Lobe | 2 | 17.7 | Tyrosinemia | + | 250 | + | Alive, 2 years post TX | Well diff HCC |
| 23 | 8 | Male | Left lobe | 1 | 2.4 | Tyrosinemia | + | 671 | + | Alive, 3 years post Tx | Well diff HCC |
| 24 | 7 | Female | Right lobe | 1 | 1.5 | Tyrosinemia | + | 908 | + | Alive, 3 years post Tx | Well diff HCC |
| 25 | 6 | Female | Right lobe | Multiple | 18 | Tyrosinemia | + | 2000 | + | ALive, 1 year post Tx | Well diff HCC |
| 26 | 10 | Female | Right lobe | 1 | 9 | - | - | 23 | - | Alive | Well diff HCC |
| 27 | 16 | Female | Right and Left lobe | Multiple | 6 | - | - | 4.4 | + | Alive, 2 years post Tx | Fibrolamellar HCC |
| 28 | 8 | Male | Left lobe | Multiple | 2.4 | Tyrosinemia | + | 3980 | + | Alive 2 years post Tx | Well diff HCC |
| 29 | 7 | Male | Left lobe | 1 | 6 | Tyrosinemia | + | 34 | - | Died | Well diff HCC |
| 30 | 13 | Female | Left lobe | Multiple | 8 | Fanconi | - | 45 | - | Died | Well diff HCC |
Different Clinicopathologic Findings of the 30 Cases of HCC in the Patients Below the Age of 18 Years
4. Discussion
Hepatocellular carcinoma is considered as a rare pediatric tumor, however, it is the second most common malignant liver tumor in this age group (6, 7). Most of HCCs in the pediatric age group has been reported in adolescents after 10 years of age, and occurrence of this tumor in young children is almost always associated with an underlying metabolic or viral liver disease (7, 8). There have not been any studies regarding HCC in the pediatric age group from Iran. All of the previous reports regarding HCC from Iran have been in the adult age group. Our center is the largest referral center of hepatobiliary surgery in the South of Iran and also the only center for pediatric liver transplantation in the country; therefore, we decided to retrieve all the cases of HCCs in the children below the age of 18 in our affiliated hospitals to find out about different clinicopathologic findings of these cases.
In the last 10 years, there have been 30 cases of HCC in the patients below the age of 18 years. Although this tumor is more common in boys, the difference in boys and girls was not significant (M/F = 1.14/1). Most of our cases have been in cirrhotic livers with known underlying cause. The most common underlying cause in our center has been tyrosinemia. Tyrosinemia is an important cause of pediatric HCC, especially in the untreated patients (8). However, we had 1 case of PFIC (Progressive familial intrahepatic cholestasis). There have been rare reports regarding the occurrence of HCC in patients with PFIC, which emphasizes on the risk of HCC in the patients with defects in bile export proteins, i.e. PFIC (9, 10).
One of our cases has been autoimmune hepatitis (AIH), under treatment that found to have HCC as well as AIH. The occurrence of HCC in AIH, especially in a young patient (17-year-old), is a rare event; however, it should be kept in mind (11).
One of our cases has been cirrhotic secondary to Alagille syndrome, which has undergone liver transplantation. There have been well-documented reports of HCC in Alagille, however, it is very rare (12).
There have been 3 cases with no underlying disease and fibrolamellar variant of HCC, which is known to occur in healthy livers of young and adolescents without cirrhosis. It used to be considered as a type of HCC with a better prognosis; however, case-control studies failed to show prognostic difference between HCC and its fibrolamellar variant (4, 13). Our patients have been 15, 11, and 16 years of age, 2 of which were unfortunately dead at the time of study. All of the other cases have been well-differentiated hepatocellular carcinoma.
Many of the previous studies have reported chronic hepatitis B related cirrhosis as the most common underlying cause of pediatric HCC (14), however, we didn’t have any case of HBV related cirrhosis in these 30 cases of pediatric HCC. We believe it is due to extensive and successful vaccination in Iran and significant decrease in the number of HBVs in the current generation of pediatric age group. With 0.96% of HBs Ag carriage, Iran is now considered as a low endemicity country for chronic infection with HBV (15).
Diagnosis of HCC is based on clinicopathologic and radiologic findings; however, AFP plays an important role in the early diagnosis and follows up of the patients with cirrhosis (16). Most of our cases had elevated AFP, which most of them were significantly high, therefore, our study confirms the value of AFP in the diagnosis of HCC even in the pediatric age group (17).
As a conclusion, HCC should be considered as a rare pediatric tumor in Iran, which is most commonly secondary to cirrhosis due to metabolic diseases specially tyrosinemia. This tumor has a poor prognosis and without liver transplantation has a short survival.