1. Background
ACLF is a unique clinical entity with high morbidity and mortality rate. It is characterized by acute onset on the chronic liver disease (CLD). The severe liver damage caused by hepatocellular insult such as hepatitis virus infection, alcohol abuse, autoimmune disease, some genetic diseases, among other causes (1). There is acute deterioration of liver function, leading to the dysfunction of one or more organs. ALCF usually involves an acute strike (2, 3), first resulting in damage to liver function, which is followed by systemic inflammatory response syndrome (SIRS), compensatory anti-inflammatory response syndrome, and finally immune paralysis (4). In cases of confirmed and unproven bacterial infections, exacerbated SIRS is very important to ACLF progression and prognosis (5). Hepatitis B virus (HBV) reactivation is the most common cause of ACLF in Asian countries (6, 7).
The inflammatory response crucial to the pathogenesis and prognosis of patients with ACLF. The pathogenic mechanism of ACLF is related to innate immunity and systemic inflammatory responses. Therefore, inhibiting innate immune activation may be a new strategy for ACLF treatment (3, 8). Usually, clinical evaluation of the inflammatory state relies on various indicators of routine peripheral blood tests, such as the white blood cell (WBC) count. In liver disease, platelet count often decreases. Increasing evidence shows that hematological parameters, such as platelet distribution width/platelet count ratio and the neutrophil-lymphocyte ratio, are predictors of endocrine disease and COVID-19 (9-11).
More than 76% of patients with liver disease suffered from thrombocytopenia (12), which may be related to liver cirrhosis, hypersplenism, and portal hypertension (13). Platelet count decrease may be the first indication of, and is often directly proportional to the severity of liver failure (14). Thrombocytopenia has multifaceted causes, such as decreased production of hormone thrombopoietin (TPO), or increased destruction of platelets in an enlarged spleen (15). TPO is mainly synthesized in hepatocytes, so it decreases when the liver cell mass is damaged or destroyed. Also, at all stages of megakaryocyte production and thrombopoiesis differentiation, only TPO can play a role in the whole process (16).
PWR has been reported to be a marker of systemic inflammatory response. Previous studies have shown that it can be used as a prognostic predictor of acute ischemic stroke and cancer (17-20). This indicates that ischemic stroke patients with a lower platelet count and a higher number of WBCs will have worse prognosis.
2. Objectives
However, there are few reports on the PWR in liver failure. In this study, we verified the relationship between PWR and prognosis, and explored the factors related to the decrease in PWR, in ACLF patients.
3. Methods
3.1. Patient Enrollment and Study Design
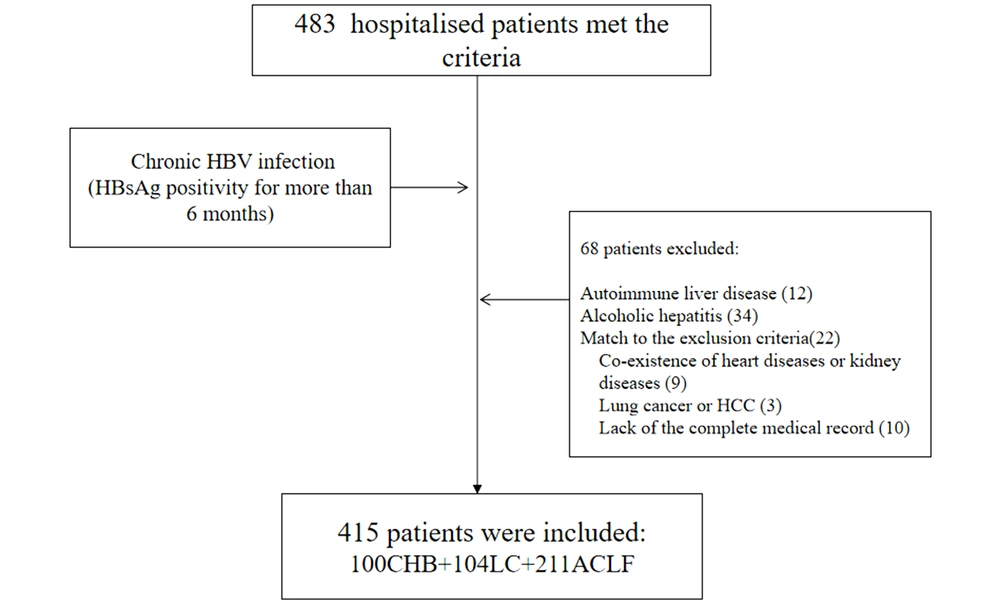
Patients with chronic hepatitis B (CHB), HBV-related liver cirrhosis (LC) and HBV-related ACLF, consecutively hospitalized at the Fifth Medical Center of the Chinese PLA General Hospital of China from January 2014 to December 2017, were selected for retrospective analysis. As shown in Figure 1, in this retrospective study, we reviewed 100 patients with CHB, 104 patients with HBV-related LC, and 211 HBV-related ACLF patients.
3.2. Data Collection
Laboratory data were recored at the time of enrollment, and included WBC count, platelet, international normalized ratio (INR), alanine transaminase (ALT), albumin (ALB), aspartate aminotransferase (AST), alpha fetoprotein (AFP), total bilirubin (TBil), and plasma thromboplastin antecedent (PTA). In the ACLF patients, we also recorded the spleen size as measured by ultrasound, infectious complications, the complication of infection, SIRS and ascites.
3.3. Definition
CHB was defined as HBV surface antigen (HBsAg)-positivity of at least 6 months’duration (21). Cirrhosis was clinically based on the patient history or ultrasonic imaging characteristics, including a small-sized liver, nodular liver surface, and splenomegaly, with or without the portal hypertension. ACLF patients met the Pacific Association for the Study of the Liver criteria (22). We excluded patients with any other serious co-existing systemic diseases and hematological diseases; with other systemic malignancies, including hepatocellular carcinoma, who were pregnant, or who lacked complete medical records. Infection status was diagnosed based on the following criteria. Spontaneous bacterial peritonitis (SBP) was diagnosed based on diagnostic paracentesis, when the neutrophil count of the ascites reached a cutoff of 250/mm3. Pneumonia diagnosis was based on symptoms and signs of an acute lower respiratory tract infection, and was confirmed by a chest radiograph showing new shadowing unrelated to any other cause. Bloodstream infection was diagnosed based on the growth of a non-common skin contaminant from ≥ 1 blood culture (BC) and of a common skin contaminant from BC of ≥ 2 samples drawn on separate sites with signs of infection. Spontaneous bacterial empyema was diagnosed based on the polymorphonuclear cell count in pleural fluid ≥ 250/mm3. Urinary tract infection was diagnosed based on the number of bacteria in the urine. For symptomatic women, a culture definition for cystitis was ≥ 102 CFU/mL of an uropathogen, and for pyelonephritis ≥ 104 CFU/mL. In non-catheter-related cystitis, counts of ≥ 102 CFU/mL are significant in urine samples obtained by catheterization. In males with cystitis, a culture of ≥ 103 CFU/mL was considered to be significant. Cellulitis was diagnosed based on the clinical signs of skin infection associated with discomfort, erythema, swelling, and warmth of the affected area. SIRS was assessed according to the recommendations of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. The diagnosis of ascites was based on ultrasound (23). The size of the spleen was measured using ultrasound imaging (iu22, Philips Healthcare, Cambridge, MA).
The study was approved by the institutional ethics committee of the Fifth Medical Center of Chinese PLA General Hospital (2019089D).
Due to the study was retrospective nature of the study and the anonymous data used, we waived the informed consent .The patient identification was anonymous.
3.4. Statistical Analysis
All statistical analyses were performed using SPSS version 19 (IBM Corp, Armonk, NY) and MedCalc software (version 15.2.2, Ostend, Belgium). Descriptive statistics were expressed as median (interquartile range, Q1 - Q3) or mean with standard deviation. The Mann-Whitney test or t-test was used to compare two continuous variables, as appropriate. The Kaplan-Meier method was used to calculate the cumulative survival rate. Multivariable Cox regression models were used to determine whether specific variables were related to the cumulative survival rate. Hazard ratios (HRs) and their 95% confidence intervals (CIs) as well as the corresponding P values are shown. The area under the receiver operating characteristic (ROC) curve of the two models was compared using the Delong test.
4. Results
4.1. Baseline Demographic, Laboratory Characteristics of the Patients
The examination indices of patients are shown in Table 1. The WBC in patients of ACLF was significantly higher than patients with CHB and LC (P < 0.0001).The platelet count differed among the three groups,being the lowest in those with ACLF, followed by those with LC patients and those with CHB (P < 0.0001).
| Variables | Chronic Hepatitis B | Liver Cirrhosis | ACLF | P Value | CHB vs LC | CHB vs ACLF | LC vs ACLF |
|---|---|---|---|---|---|---|---|
| Gender (male) | 66 (66.0) | 70 (67.3) | 176 (83.4) | 0.0001 | 0.843 | 0.001 | 0.001 |
| Age (y) | 43.55 ± 10.33 | 47.45 ± 10.12 | 44.14 ± 11.27 | 0.013 | 0.006 | 0.657 | 0.01 |
| WBC (× 109/L) | 5.58 ± 1.44 | 4.99 ± 1.65 | 7.45 ± 3.87 | 0.0001 | 0.026 | 0.0001 | 0.0001 |
| PLT (× 109/L) | 187 (149, 217) | 128 (103, 171) | 86 (55, 120) | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| ALT (U/L) | 27 (18, 40) | 24 (19, 30) | 154 (72, 466) | 0.0001 | 0.1 | 0.0001 | 0.0001 |
| AST (U/L) | 24 (20, 35) | 25 (21, 31) | 173 (104, 366) | 0.0001 | 0.585 | 0.0001 | 0.0001 |
| TBil (µmol/L) | 10.7 (8.32, 14.08) | 10.9 (7.83, 14.27) | 287.2 (200.35, 393.55) | 0.0001 | 0.988 | 0.0001 | 0.0001 |
| ALB (g/L) | 41.14 ± 3.89 | 41.07 ± 5.04 | 29.11 ± 4.77 | 0.0001 | 0.972 | 0.0001 | 0.0001 |
| INR | 0.90 ± 0.049 | 0.94 ± 0.043 | 2.05 ± 0.62 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| PTA (%) | 153.8 ± 10.69 | 106.2 ± 8.92 | 33.54 ± 8.86 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| AFP | 2.14 (1.51, 3.34) | 2.22 (1.58, 3.61) | 65.00 (17.55, 239.95) | 0.0001 | 0.642 | 0.0001 | 0.0001 |
| PWR | 32.65 (25.68, 43,05) | 27.82 (20.72, 34.94) | 13.33 (9.01, 18.50) | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
Abbreviations: WBC, white blood cell; PLT, platelet; ALT, alanine transaminase; AST, aspartate amino transferase; TBil, total bilirubin; ALB, albumin; PTA, Prothrombin activity; AFP, alpha fetal protein; PWR, platelet to white blood cell.
a P value estimated by one-way analysis of variance (ANOVA), t-test, or the Kruskal-Wallis H test for continuous data, and chi-square test for categorical data.
The median PWR of patients with ACLF was 13.33, which was significantly lower than that of other patients (P < 0.0001). Other laboratory parameters such as ALB, ALT, AST, TBiL, INR and PTA levels in patients with ACLF were significantly difference compared with others (P < 0.05). The ALB, PTA values were significantly reduced in ACLF patients. The remaining indicators were significantly higher in ACLF patients.
By radiography, 127 patients has splenomegaly, and 150 ACLF patients had ascites. Of all the ACLF patients, 65 patients had complications related to infection at intake, and 14 patients had SIRS.
4.2. Association of PWR with 1-Month Mortality in HBV-Related ACLF
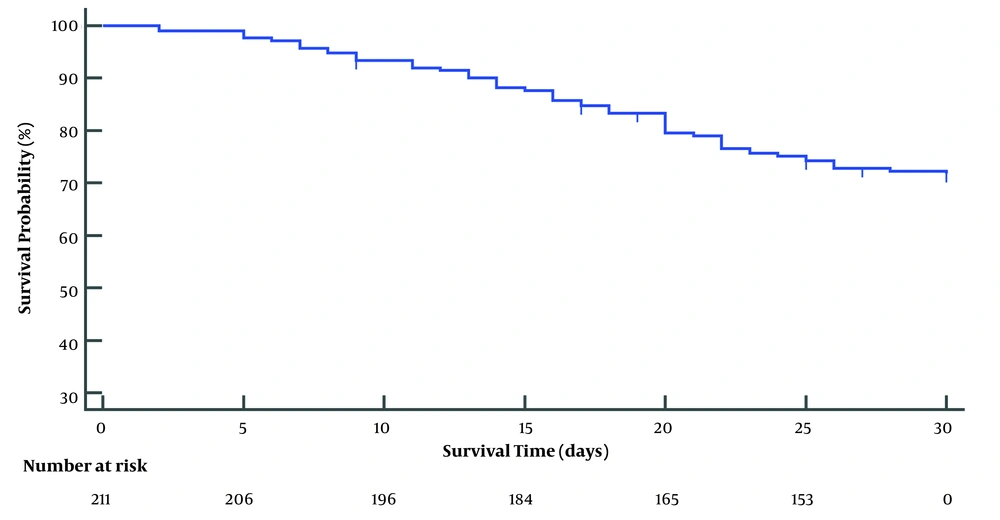
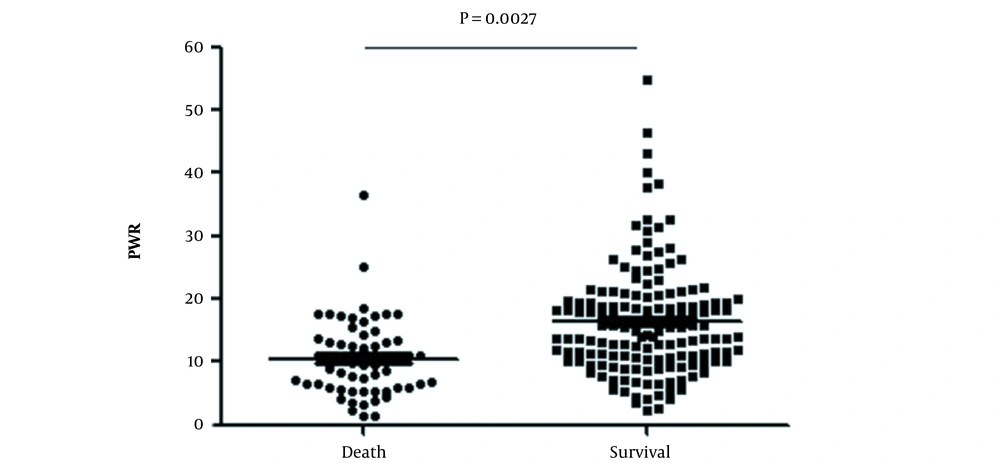
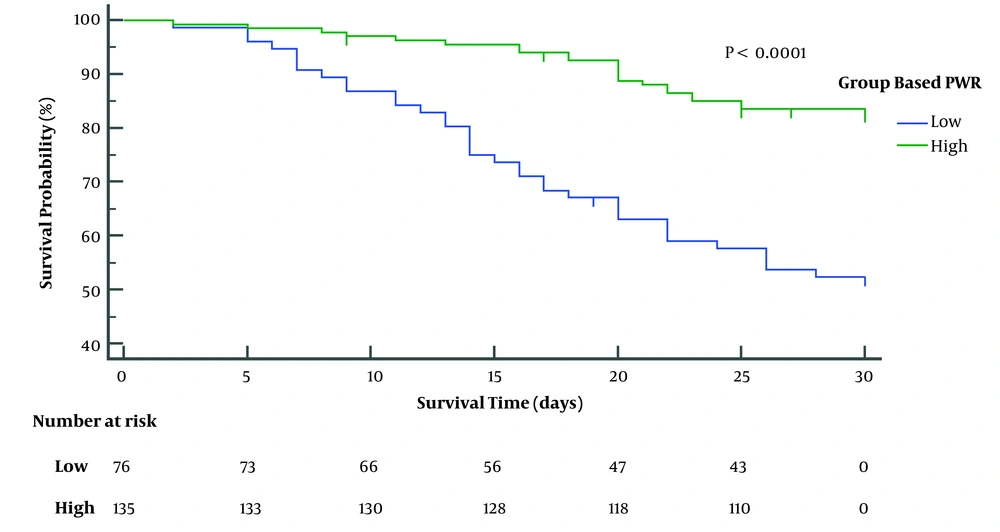
The median follow-up period was 30 days. Fifty-nine ACLF patients died within 1 month, the non-liver transplant 1-month survival rate of ACLF was 71.8% (Figure 2). Upon dividing patients into survival and decesed groups, the PWR in survivors was markedly higher than that in deceased patients (P = 0.0027) (Figure 3). The Cut-off value of the continuous variables, such as age, WBC, creatinine (Cr), PWR, TBil, monocyte count, sodium (Na), prothrombin activity (PA), INR, ALT, AST, and ALB were calculated using ROC curve. By univariate analysis, 12 factors reached statistical significance with patient survival: age, Cr, PWR, TBiL, monocyte, cholesterol, triglycerides, Na, PTA, INR, SBP (Table 2).
| Variables | Cases | One-Month Survival Rate (%) | χ2 Value | P-Value |
|---|---|---|---|---|
| Age (y) | 3.57 | 0.044 | ||
| ≤ 38 | 70 | 79.5 | ||
| > 38 | 141 | 67.9 | ||
| Cr | 9.14 | 0.002 | ||
| ≤ 118 | 181 | 74.9 | ||
| > 118 | 30 | 52.8 | ||
| PWR | 25.19 | 0.0001 | ||
| ≤ 10.76 | 76 | 52.3 | ||
| > 10.76 | 135 | 82.8 | ||
| TBiL | 12.15 | 0.0001 | ||
| ≤ 384 | 151 | 78.0 | ||
| > 384 | 60 | 55.7 | ||
| Monocyte | 8.72 | 0.003 | ||
| ≤ 1.27 | 189 | 75.2 | ||
| > 1.27 | 22 | 49.0 | ||
| Cholesterol | 18.86 | 0.0001 | ||
| ≤ 0.96 | 53 | 49.9 | ||
| > 0.96 | 158 | 79.0 | ||
| Triglycerides | 6.69 | 0.01 | ||
| ≤ 0.93 | 90 | 61.9 | ||
| > 0.93 | 121 | 79.1 | ||
| Na | 10.69 | 0.001 | ||
| ≤ 135.5 | 99 | 61.2 | ||
| > 135.5 | 112 | 81.1 | ||
| PTA | 30.00 | 0.0001 | ||
| ≤ 25.4 | 38 | 39.1 | ||
| > 25.4 | 173 | 79 | ||
| INR | 13.95 | 0.0001 | ||
| ≤ 2.12 | 142 | 79.6 | ||
| > 2.12 | 69 | 55.1 | ||
| Infection | 4.47 | 0.035 | ||
| No | 61 | 81.7 | ||
| Yes | 150 | 67.8 |
Abbreviations: Cr, creatinine; TBil, total bilirubin; PTA, Prothrombin activity; AST, aspartate amino transferase.
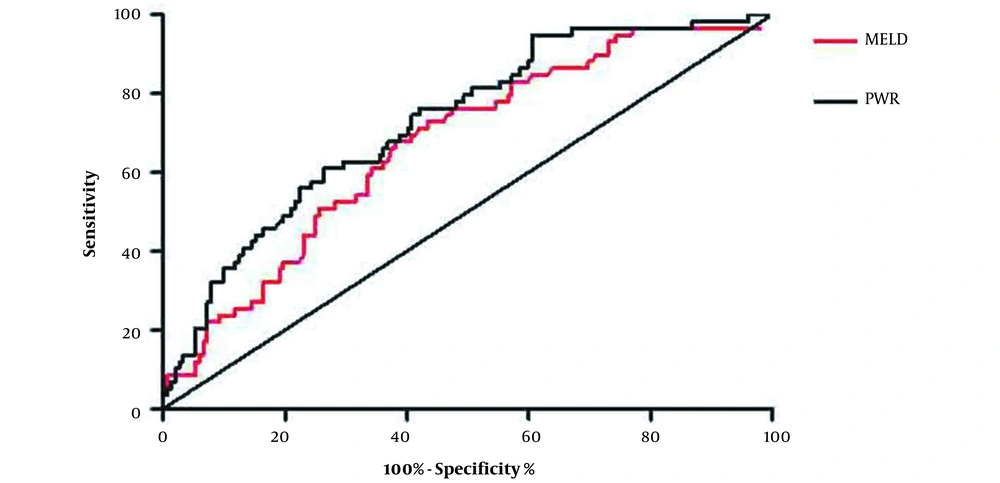
Then, multivariable Cox regression analysis (with outcome as the dependent variable) was used to determine the independent variables associated with the outcome. Using backward Cox regression analysis, the independent factors associated with mortality were PWR, age, TBIL, PA, and AST (Table 3). To evaluate the predictive value of PWR in the one-month survival rate of patients with ACLF, we used ROC curve analysis. As shown in Figure 4, the area under the ROC curve for PWR between survival and deceased patients was 0.728 [95% confidence interval (CI): 0.655 - 0.801; P < 0.0001). Based on the Youden index, the optimal cut-off value was 10.76 (sensitivity: 73.7%; specificity: 61.0%; Youden’s index: 0.347). However, the area under the ROC curve for the model for end-stage liver disease (MELD) was 0.672 (95% CI, 0.593 - 0.750; P = 0.0001). The optimal cut-off value was 29.64 (sensitivity: 67.8%; specificity: 61.8%). While the p value of the AUC area between PWR and MELD was 0.036. Using this cut-off value, mortality rates were higher in the high than in the low PWR group (Figure 5) (P < 0.0001).
| Predictor | HR | 95% CI | P |
|---|---|---|---|
| PWR | 0.900 | 0.853 - 0.949 | < 0.0001 |
| Age (y) | 1.035 | 1.010 - 1.062 | 0.007 |
| TBIL (umol/L) | 1.002 | 1.001 - 1.004 | 0.013 |
| PTA (%) | 0.942 | 0.915 - 0.969 | 0.013 |
| AST | 1.001 | 1.000 - 1.001 | 0.017 |
Abbreviations: TBil, total bilirubin; PTA, prothrombin activity; AST, aspartate amino transferase; PWR, platelet to white blood cell.
4.3. Factors Affecting the PWR Value in Patients of ACLF
The PWR value was identified as an independent risk factor for survival in ACLF patients. There was a significant difference in PWR between ACLF and other patients. We then attempted to identify factors affecting PWR value. We included plausible factors, i.e., cirrhosis, splenic vein diameters, spleen size and the diameter, ascites, SIRS and infection. Logistic regression analysis revealed ascites (P = 0.003) and infection (P = 0.003) were independent factors associated with the reduction of PWR, as shown in Table 4.
| Predictor | Univariate Analysis HR (95% CI) | P | Multivariate Analysis; HR (95% CI) | P |
|---|---|---|---|---|
| Ascites | ||||
| No | 1 | |||
| Yes | 0.25 (0.118 - 0.529) | < 0.001 | 0.31 (0.142 - 0.677) | 0.003 |
| Infection | ||||
| No | 1 | |||
| Yes | 0.25 (0.135 - 0.463) | < 0.001 | 0.363 (0.187 - 0.703) | 0.003 |
5. Discussion
In this study, we verified the relationship between PWR and prognosis, and explored the factors related to the PWR decrease in ACLF patients. At baseline, the platelet count in patients with HBV-related ACLF was significantly lower than that in CHB and LC patients. The PWR was markedly higher in ACLF survivors than in ACLF patients who died. PWR, age, total bilirubin, prothrombin activity, and aspartate transaminase were independent predictors of the 30-day survival rate of ACLF patients. We also found that ascites and infection were independent factors related to PWR decrease in ACLF patients.
ACLF patients have severe disease, rapid progression, a high incidence of complications, and a high short-term mortality rate (24). Because of this high mortality, predicting the prognosis of liver failure is important for patient management. Recently, numerous studies have evaluated models to predict the prognosis of patients with ACLF, using Child-Turcotte-Pugh scores, including ALB, TBil, prothrombin (PT), ascites, and hepatic encephalopathy, MELD scores, including TBil, PT-INR, serum Cr, etiology, and MELD-Na scores. However, the score calculation of these scores is more complicated; thus, a simpler score is needed to predict the prognosis of ACLF patients.
In many diseases, platelet count is markedly reduced (25, 26). Thrombocytopenia is regarded as a poor prognostic sign in patients admitted to the intensive care unit with various diseases (27) and is common in patients with CLD. It increases the bleeding risk, prolonging hospital stay and increasing hospitalization costs. We found that platelet counts in ACLF patients was significantly lower than that in patients with CHB. Thrombocytopenia in liver diseases may be due to splenomegaly, decreased activity of platelet-promoting hormone, and bone marrow suppression caused by chronic viral infection (28-30). Additionally, decreased platelet production, antiplatelet antibodies, diffuse intravascular coagulation, translocation toxins, and intestinal-derived substances may cause low platelet counts (31). Recently, there is an additional mechanism for increased platelet destruction in CLD patients, which is the effect of abnormal rheological conditions caused by elevated portal pressure (32). Platelets are not only essential for hemostasis, but also promote liver regeneration and prevent the progression of liver fibrosis by secreting a variety of growth factors, such as platelet-derived growth factor and hepatocyte growth factor (33). As a feedback response, thrombocytopenia can further aggravate liver damage and promote the progression of CLD and liver cirrhosis. In addition, in CLD patients, platelet transfusion and splenectomy are used as platelet augmentation therapy to improve liver function (34). Vinholt et al. found that the lower the platelet count, the more severe the liver cirrhosis and platelet function impairment (35). Therefore, the platelet index is of great significance in CLD.
When studying the relationship between platelets and the outcome of patients with severe disease, a previous study found that the platelet count was increased in survivors but not in non-survivors after ICU admission for various diseases (36). A dynamic change in platelets indicates a change in liver disease severity. Our univariate analysis showed that a dynamic process of platelet change is closely related to patient prognosis. In previous studies, the PWR ratio was associated with the prognosis of patients with acute stroke and acute coronary syndrome (20, 37). ACLF patients are prone to infection, resulting in elevated WBCs (38); therefore, due to an increase in WBCs and a decrease in platelets, the PWR will eventually decrease. We also found that the PWR was markedly higher in survivors than in those who died. Since routine blood examination is a simple detection method, the PWR can provide clinicians with a quick reference, within 30 min. Accurately predicting short-term survival time of ACLF patients is helpful to make appropriate medical decisions to improve the survival rate. Furthermore, we found that the combination of PWR, age, TBil, PA, and AST could predict the 1-month survival rate.
Kamath et al. (39) first reported the MELD score, in 2001, as a means to assess the prognosis of patients after transjugular portosystemic shunts. It was later confirmed to be suitable for evaluating severity of various CLDs, predicting the survival rate of patients after liver transplantation, and is widely used as a scoring system for organ allocation in liver transplantation. However, the MELD score does not consider inflammation, which significantly influences prognosis. We found that PWR was strongly correlated with ACLF prognosis. The PWR calculation requires the assessment of two simple factors, making it simpler to determine than the MELD score.
We also found that ascites and infection were related to the PWR. Infection increases the WBC, which reduces the PWR value. Immune function is severely damaged in ACLF patients, resulting in infection (40, 41). The presence of various pro-inflammatory and anti-inflammatory factors can be detected in ACLF patients, such as sTNF-aR2,TNF-a, sTNF-aR1,IL-2R, IL-2, IL-6, IL-8, IL-10 (42-44). High levels of serum IL-6 increase the mortality of HBV-associated ACLF. ACLF is characterized by severe systemic inflammation, which is associated with its poor prognosis (45). Ascites was related to the severity of portal hypertension and hypoproteinemia, further demonstrating the extent of cirrhosis. Thus, ascites and infection lowered the PWR value in ACLF patients, and the lower the PWR value, the lower was the survival rate.
One important limitation of our study is that it was a retrospective analysis with a potential selection bias. The cases were from a single center, which is another limitation. Another limitation is that these patients have only been followed up to 2017. Regardless of the above limitations, PWR is an easy-to-calculate indicator when compared to MELD or MELD-Na. In other words, when a patient is admitted to the hospital, PWR can quickly identify the severity of the patients.
5.1. Conclusions
In conclusion, we here demonstrated the clinical significance of PWR in patients with ACLF. The PWR value, which is easy and convenient to calculate, may be an important predictor of the prognosis of ACLF patients.