1. Background
Hepatitis B virus (HBV) infection is a life-threatening public health problem affecting over 250 million people worldwide (1). The prevalence of HBV infection has a range of 0.1%-20% in different geographic regions of the world (2). Epidemiological studies in Turkey have shown that approximately 4% of the population is positive for hepatitis B surface antigen (HBsAg) (3, 4). Current treatment options for chronic hepatitis B (CHB) include interferon and oral antiviral alternatives. Viral suppression achieved by nucleos(t)ides analogs (NUCs) reduces complications that cause mortality and morbidity, especially cirrhosis and hepatocellular carcinoma. However, achieving these aims requires a life-lasting treatment in the majority of patients because HBsAg loss is a rare event in CHB patients undergoing NUCs (5).
Tenofovir is a NUC that inhibits HBV-DNA polymerase. Tenofovir has two formulations used in the treatment of CHB, namely tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide fumarate (TAF) (6). Adverse effects, such as reduced bone mineral density (BMD) and renal toxicity, have been reported for long-term TDF use (5, 7). TAF reaches the hepatocytes intact because it has a longer plasma half-life and higher plasma stability than TDF. These properties allow TAF to reach the same efficacy as TDF with a smaller dose. Moreover, bone and renal-related side effects are less frequent due to low systemic exposure (8, 9). Entecavir (ETV) is a NUC that selectively inhibits HBV replication and is an influential treatment option in CHB. It is generally well-tolerated, and if necessary, renal dose adjustment is possible (7). Switching from TDF to TAF or ETV is recommended for patients with a high potential for bone and renal-related side effects and those with bone and renal effects due to long-term treatment (10, 11).
2. Objectives
The present study aimed to compare the changes in treatment efficacy and side effects within the first 6 months of switching from TDF to ETV or TAF in CHB patients.
3. Methods
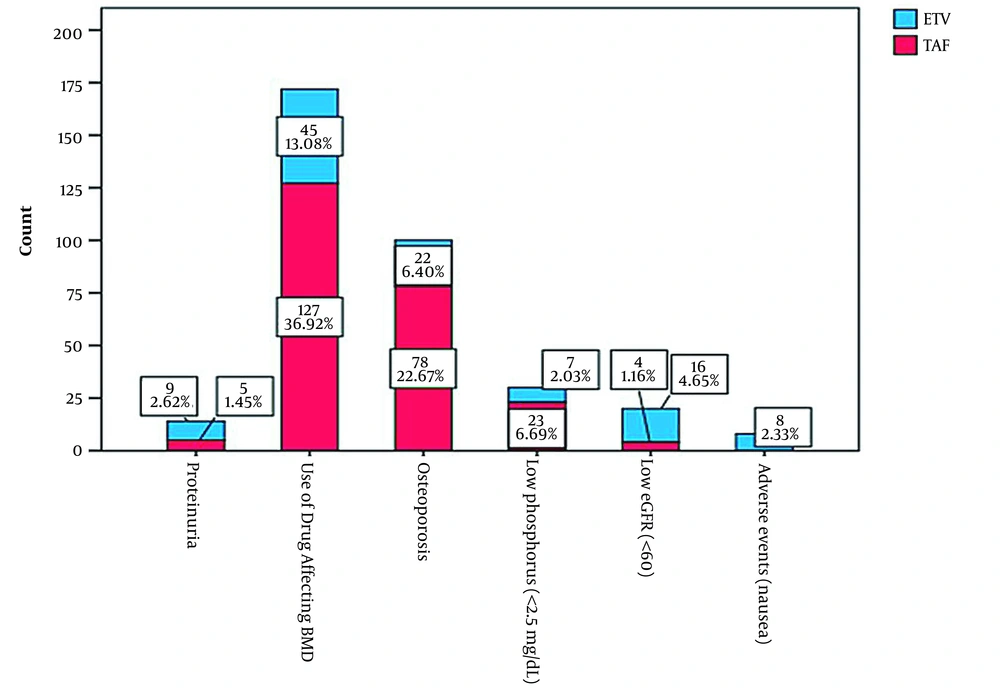
This retrospective cohort study was conducted in infectious diseases and gastroenterology clinics in tertiary care centers in the Eastern and Southeastern Anatolia regions of Turkey, where CHB is almost endemic. Centers were invited by E-mail to participate in this investigation. Of 17 invited tertiary care centers, ten hospital-based clinics agreed to participate in the study. The study population included patients referring to these centers during January 1, 2019-December 31, 2019. The inclusion criteria were being over 18 years old, having used TDF due to CHB, and having the indications to switch from TDF to TAF or ETV. These indications are based on the recommendations in the European Association for the Study of the Liver (EASL) 2017 HBV infection management guidelines. The indications entail proteinuria, a history of using a drug that affects BMD, osteoporosis, phosphorus level < 2.5 mg/dL, chronic steroid use, a history of atraumatic bone fracture, estimated glomerular filtration rate (eGFR) < 60, being under dialysis therapy, a history of renal transplantation, and the presence of adverse effects (11).
A total of 344 patients who were changed to TAF or ETV by discontinuing TDF according to the inclusion criteria were included in the study. The ETV and TAF groups consisted of 107 and 237 patients, respectively. The treatment of patients with ETV or TAF was based on the discretion of their physicians. Demographic and clinical data of the patients, including gender, age, diabetes mellitus, hypertension, cirrhosis diagnosed based on pre-treatment hepatic biopsy, a family history of hepatitis B, TDF usage time, and body mass index, were recorded and analyzed.
The serologic laboratory results were recorded on anti-delta, anti-hepatitis C virus, anti-human immunodeficiency virus (HIV), and HBV DNA levels. A patient was determined to have alanine aminotransferase (ALT) normalization if ALT was ≤ 35 IU/L for men and ≤ 25 U/L for women according to the American Association for the Study of Liver Diseases (AASLD) (12). The HBV DNA < 20 UI/mL was defined as undetected HBV DNA. Renal function was assessed based on serum creatinine, serum phosphorus, and eGFR calculated by the modification of diet in the renal disease study equation.
Values at baseline and month 6 of TAF and ETV treatment were obtained for white blood cell and platelet counts, ALT (U/L), aspartate aminotransferase (AST; U/L), phosphorus (mg/dL), total cholesterol (TC; mg/dL), low-density lipoproteins cholesterol (LDLC; mg/dL), high-density lipoproteins cholesterol (HDLC; mg/dL), triglycerides (mg/dL), international normalized ratio, total bilirubin (mg/dL), creatinine (mg/dL), albumin (g/dL), alpha-fetoprotein (U/L), and eGFR. Baseline and 6-month hip and spine T-scores were evaluated to follow-up BMD in the patients. Previous pharmacovigilance reports were analyzed when assessing adverse drug effects. All statistical analyses were performed using IBM SPSS Statistics version 21.0 (IBM Corp., Armonk, NY, US) with a P-value < 0.05 accepted as the level of significance. Ethics approval was obtained from the local Ethics Committee on February 3, 2020 (number 37732058-514.10).
3.1. Statistics
The sample size was calculated based on the assumption that the change from TDF to TAF or ETV would have an indifferent effect on renal functions. The GFR values after at least 6 months of follow-up from the literature were used to calculate the minimum required number of samples in each group. The sample size was obtained as 102 in each group, considering a power of 80%, a confidence level of 95%, and a margin of 5 units (13, 14). Descriptive data were presented as mean, standard deviation, median, minimum, maximum, number, and percentage. The normality of data distribution was assessed using the Kolmogorov–Smirnov test. In addition, the Chi-square and Mann-Whitney U tests were used for making comparisons between the two groups. Changes in markers in the first 6 months were evaluated by subtracting the baseline values from 6-month values. The Mann-Whitney U test was used to compare the values of baseline and 6 months in the treatment arms. As appropriate, repeated measures were compared using Wilcoxon Signed Rank and McNemar tests.
4. Results
4.1. Baseline Characteristics
A total of 344 patients who were changed from TDF to ETV (n = 107, 31.1%) or TAF (n = 237, 68.9%) were included in the study. The mean age of patients was 41.14±13.46 years, and 224 (65.1%) of participants were male. The indications for switching from TDF therapy to TAF or ETV are presented in Figure 1, and the two most common causes were the use of medicines affecting BMD and osteoporosis. The other switching indications were low phosphorus, low eGFR, proteinuria, and nausea. The distribution of basic characteristics at ETV and TAF therapy initiation was similar between the two groups (Table 1).
| Variables | ETV (n = 107) | TAF (n = 237) | P-Value c |
|---|---|---|---|
| Age (y) | 38 (33 - 51) | 38 (33 - 51.5) | 0.608 |
| Gender, male | 64 (59.8) | 160 (67.5) | 0.166 |
| Cirrhosis | 9 (8.4) | 12 (5) | 0.099 |
| Blood hypertension | 10 (9.3) | 22 (9.3) | 0.964 |
| Diabetes mellitus | 9 (8.4) | 12 (5.1) | 0.221 |
| Family history of HBV | 54 (50.5) | 132 (55.7) | 0.171 |
| TDF usage time (mon) | 36 (24 - 48) | 30 (24 - 48) | 0.56 |
| BMI (kg/m2) | 27 (25 - 29.3) | 26.8 (24.3 - 29.3) | 0.474 |
| Anti-HCV antibody | 1 (0.9) | 7 (3) | 0.087 |
| Anti-HIV antibody | - | - | - |
| Anti-HDV antibody | 12 (11.2) | 31 (13.1) | 0.843 |
| Undetectable HBV DNA | 91 (85) | 215 (90.7) | 0.12 |
| Platelet count (109/L) | 222 (184 - 265) | 219 (174 - 267) | 0.547 |
| ALT (U/L) | 33 (22 - 44) | 33 (21 - 44) | 0.999 |
| AST (U/L) | 29 (23 - 37) | 31.5 (24 - 37) | 0.586 |
| Phosphorus (mg/dL) | 3 (2.6 - 3.3) | 3 (2.6 - 3.3) | 0.805 |
| TC (mg/dL) | 188 (178 - 210) | 194 (178 - 212) | 0.578 |
| LDLC (mg/dL) | 102 (92 - 115) | 101 (92 - 112) | 0.79 |
| HDLC (mg/dL) | 55 (44 - 65) | 55 (45 - 65) | 0.561 |
| Triglycerides (mg/dL) | 186 (167 - 201) | 192 (132 - 211) | 0.131 |
| INR | 1.1 (1 - 1.23) | 1.14 (1.08 - 1.22) | 0.345 |
| Total bilirubin (mg/dL) | 0.9 (0.56 - 1.2) | 0.99 (0.67 - 1.2) | 0.084 |
| Creatinine (mg/dL) | 0.9 (0.74 - 1.1) | 0.9 (0.77 - 1.09) | 0.284 |
| Albumin (g/dL) | 3.55 (3.2 - 4.3) | 3.45 (3.2 - 4.4) | 0.439 |
| AFP (mg/dL) | 2.9 (1.7 - 3.32) | 2.9 (1.85 - 3.32) | 0.253 |
| GFR (mL/min/1.73 m2) | 99 (87 - 108) | 101 (93 - 111) | 0.127 |
| Hip T-score | -2 (-2.7 - -2) | -2 (-2.8 - -1.5) | 0.237 |
| Spine T-score | -1.6 (-2 - -1.1) | -1.5 (-2 - -1) | 0.671 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; TC, total cholesterol; LDLC, low-density lipoprotein cholesterol; HDLC, high-density lipoprotein cholesterol; INR, international normalized ratio; AFP, alpha-fetoprotein; GFR, glomerular filtration rate.
a Bone mineral density in the lumbar spine and a hip region close to the hip joint was calculated using dual-energy X-ray absorptiometry (DEXA).
b Values are expressed as No. (%) or median (IQR).
c Chi-square test and Mann-Whitney U test.
4.2. Virologic and Biochemical Response in Patients Switched from TDF to TAF or ETV
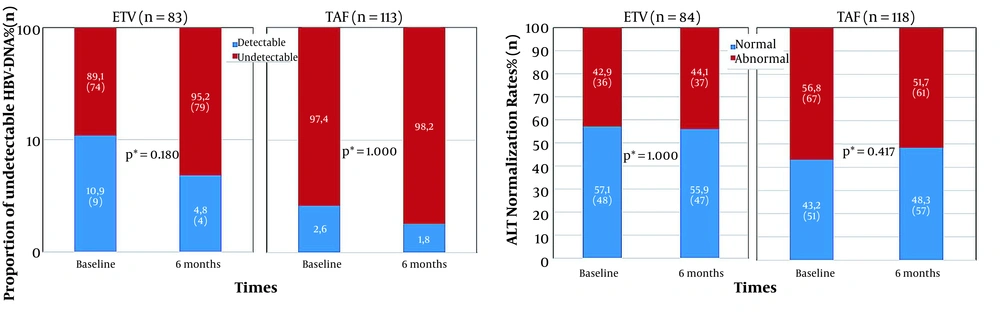
The HBV-DNA of 196 patients was evaluated both at the beginning and after 6 months. Among these patients, 83 (42.3%) cases were in the ETV group, and 113 (57.6%) were in the TAF group. Virological and biochemical response results from baseline to the sixth month in the ETV and TAF groups are presented in Figure 2. The median duration of TDF treatment for those with detectable HBV-DNA at baseline was 12 months. The proportion of undetectable HBV-DNA increased from 89.1 to 95.2% in those who switched to ETV treatment and 97.2 to 98.2% in those who switched to TAF treatment. However, this increase in the proportion of undetectable HBV-DNA in the sixth month compared to the baseline in both treatment groups was not statistically significant (P = 0.18 and P = 1, respectively). No statistically significant difference was found between the proportion of undetectable HBV-DNA of the two treatment groups in month six (P = 0.221) (Figure 2).
ALT levels were evaluated at baseline and after 6 months in 84 patients switched from TDF to ETV, and 118 patients switched to TAF. ALT normalization percentage declined from 57.1 to 55.9% and elevated from 43.2 to 48.3% in those who switched to ETV and TAF treatments, respectively. No significant difference was found in ALT normalization in the ETV and TAF groups in the sixth month compared to the baseline levels (P = 1 and P = 0.417, respectively). There was no statistically significant difference between the sixth-month ALT normalization proportion of the two treatment groups (P = 0.284) (Figure 2).
In patients with cirrhosis, the proportion of undetected HBV-DNA rose from 77.8 to 83.3% in the ETV group and from 91.7 to 100% in the TAF group (P = 0.551). The patients with hepatitis delta co-infection had no difference in undetectable HBV-DNA rates with 83.3 and 93.5% in the ETV and TAF groups, respectively (P = 1). Positivity for HBeAg was detected in 79 (22.4%) cases. HBV-DNA was assessed in 31 patients of the TAF group and 19 patients of the ETV group both at the baseline and after 6 months. In these TDF-experienced patients, only one person in the TAF group and two patients in the ETV group had detectable HBV-DNA at baseline and after 6 months.
4.3. Renal and Bone Results of Patients Who Switched from TDF to TAF and ETV
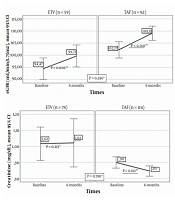
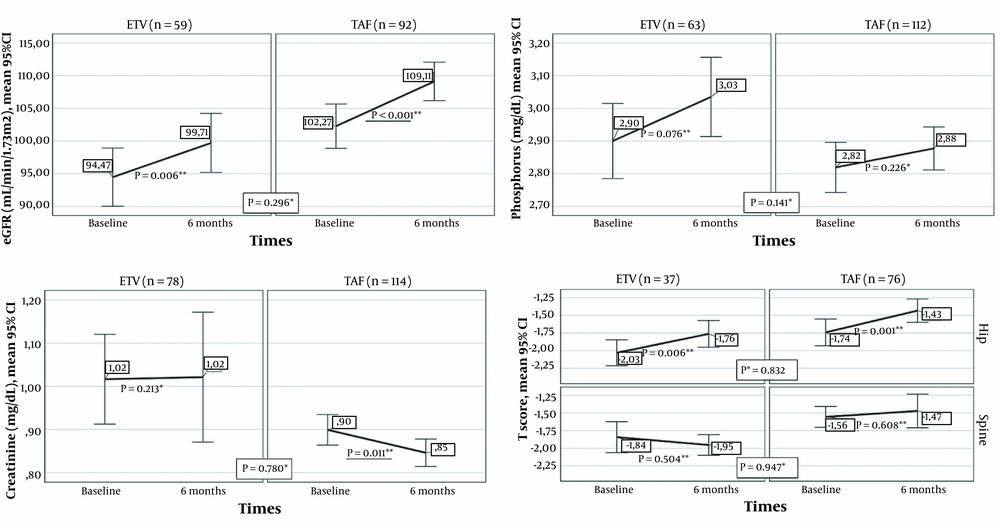
Renal and bone results from baseline to the sixth month in the ETV and TAF groups are presented in Figure 3. The eGFR mean augmented significantly in the sixth month compared to the baseline in patients who switched from TDF to ETV, as well as in individuals who switched from TDF to TAF (P = 0.006 and P < 0.001, respectively). Creatinine levels diminished significantly in the sixth month compared to the baseline levels among patients who switched to TAF, whereas no significant difference was found in the ETV group (P = 0.011 and P = 0.213, respectively). In addition, no significant change was noted in either ETV or TAF groups in terms of phosphorus (P = 0.076 and P = 0.226, respectively). No significant difference was found between the ETV and TAF groups regarding the alterations in the serum values of eGFR (P = 0.296), creatinine (P = 0.78), and phosphorus (P = 0.141).
Renal and bone results from baseline to month six in the ETV and TAF groups (* Comparison of changes in markers in the first six months between treatment groups; Mann-Whitney U test, ** Comparison of the values of the markers at baseline and the sixth month in the treatment arm, Wilcoxon Signed-Ranks Test).
Thirty-seven (34.5%) of those who received ETV treatment and 76 (32%) of patients in the TAF group were assessed for BMD at baseline and in the sixth month. In both treatment arms, the mean hip T-score increased significantly in the sixth month compared to the baseline (ETV: P = 0.006 and TAF: P = 0.001). On the other hand, spine T-scores of the two treatment arms were not significantly different at the sixth month compared to the baseline (ETV: P = 0.504; TAF: P = 0.608). Moreover, no significant difference was observed between the ETV and TAF groups in terms of changes in the mean hip T-score and mean spine T-score in the first six months (P = 0.947 and P = 0.832, respectively).
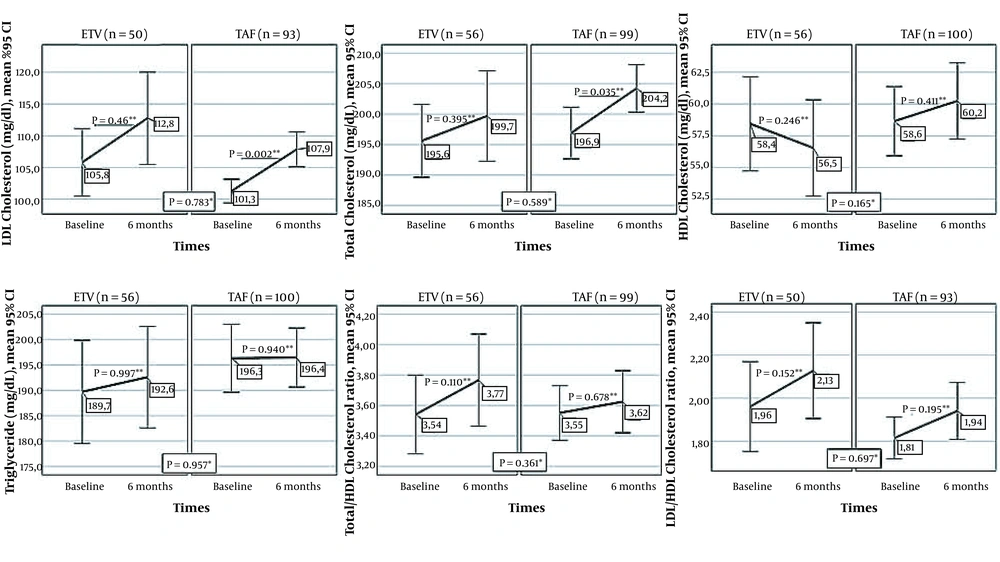
4.4. Changes of Lipid Profile in the First Six Months in Patients who Switched from TDF to TAF and ETV
The lipid profile of patients who switched from TDF to TAF and ETV at baseline and sixth month is shown in Figure 4. There was no significant difference between the baseline and 6th-month levels of HDLC, triglyceride, LDLC/HDLC, and the TotalC/HDLC ratios of cases who switched to ETV or TAF. In patients who switched to TAF or ETV, LDLC was significantly higher in the sixth month than baseline values (P = 0.002 and P = 0.049, respectively). TC increased significantly in the TAF group (P = 0.035). No significant difference was found between TAF and ETV treatments in terms of changes in the serum values of TC (P = 0.589), LDLC (P = 0.783), HDLC (P = 0.165), triglyceride (P = 0.957), and the proportion of LDLC/HDLC (P = 0.697), TC/HDLC (P = 0.361).
Changes in lipid profile between the onset of ETV and TAF treatment and month six of treatment (* Comparison of changes in markers in the first six months between treatment groups; Mann-Whitney U test, ** Comparison of the values of the markers at baseline and the sixth month in the treatment arm, Wilcoxon Signed-Ranks Test).
4.5. Adverse Effects and Treatment Modification
No side effect was reported in any of the patients in the ETV group after switching from TDF therapy, while 10 (4.4%) of those who switched to TAF treatment reported adverse effects. The most common adverse effects of TAF therapy were hair loss in five patients (2.1%) and fatigue in three patients (1.3%). Treatment was changed again in one patient due to the adverse effects of TAF (hair loss) and in one other patient due to unresponsiveness to TAF.
5. Discussion
Maintaining treatment efficacy and monitoring the side effects are the most critical points in the long-term treatment management of all chronic diseases. The NUCs with high barrier resistance, including ETV, TDF, and TAF, became first-line agents in the treatment of CHB due to the side effects of interferon-based therapies (15, 16). However, NUC therapy can be stopped when the seroclearance of HBsAg is achieved. Furthermore, the indications of NUC discontinuation are limited. Consequently, it is recommended to be continued for a long time in most patients (11). In the present study, the efficacy and safety of TAF and ETV were compared during the first 6 months in CHB patients with TDF experience.
The virological response in HBV infection aims to obtain an undetectable HBV-DNA level without negative consequences, such as cirrhosis and HCC. In a randomized controlled study evaluating CHB patients who received TDF treatment for more than 10 years, virological response rates were found to be 100% for HBeAg-negative patients and 98% for HBeAg-positive patients. The median duration of TDF treatment before the treatment switch was 30 months. Therefore, the initial proportion of undetectable HBV-DNA in these TDF-experienced patients was high in both treatment groups (17). In previous randomized controlled trials and real-life cohorts, the viral suppression rates of ETV and TAF were found to be similar to TDF (18-21).
The literature comparing ETV and TAF is limited. Half of the patients with ETV experience were switched to TAF in a study, and the groups were compared. No significant difference was found between the two groups after 24, 48, and 96 weeks in serum HBsAg levels (22, 23). In our study, the median duration of TDF treatment for those with detectable HBV-DNA at baseline was 12 months. The proportion of undetectable HBV-DNA elevated from 89.1 to 95.2% in the ETV group and 97.2 to 98.2% in the TAF group. However, the two groups were not significantly different. Our findings showed that the virological response provided by TDF was persistent.
ALT normalization has been defined as an additional endpoint associated with a reduction in viral replication and necroinflammation under treatment (11). In a study comparing TDF and TAF treatments, the proportion of ALT normalization was found to be significantly higher in those receiving TAF (19). In real-life cohorts, ALT normalization was also higher in patients who switched from TDF to TAF (24). In a meta-analysis of randomized controlled trials, the difference in the percentage of ALT normalization at 144 weeks between TDF and ETV in treatment-naive patients was not significant (25). We found a partial reduction in ALT normalization after a switch to ETV. The ALT normalization decreased from 57.1 to 55.9% in patients who switched to ETV treatment and increased from 43.2 to 48.3% in those who switched to TAF. There were no significant differences between the ALT normalization proportion of the ETV and TAF groups in the sixth month compared to the baseline levels. Although no study has compared ALT normalization between ETV and TAF, in a study, a significant increase was observed in week 24 after the transition to TAF in patients with a median of 5 years of receiving ETV (26). We suggested evaluating ALT normalization over longer observation periods.
TDF is actively secreted by the kidneys via organic anion transporters (i.e., OAT1 and OAT3), causing the exposure of the proximal renal tubules to high concentrations of tenofovir (9, 27, 28). In contrast, TAF is not a substrate of renal OATs and does not show OAT-dependent cytotoxicity (28). After switching from TDF to TAF, rapid recovery of lost renal function has been demonstrated with increased eGFR (24). A study revealed that eGFR declined significantly after two years of treatment in the TDF group, while in the ETV group, eGFR was stable for the first three years and improved significantly in years 4 and 5 (29). Consistent with the literature, in our study, eGFR augmented significantly in the sixth month in patients who switched from TDF to ETV and TDF to TAF. The absence of a significant difference between ETV and TAF therapy in terms of eGFR in month 6 of treatment supported the previous investigations (22).
On the other hand, in the sixth month, creatinine levels diminished significantly among patients who switched to TAF, whereas no significant difference was observed in those who switched to ETV. The latter result might be due to the metabolization of TDF and ETV by the kidneys, the excretion of TAF mainly in the feces (37.1%), and a very small amount of TAF secreted by the kidneys (< 1%) (30). There was no difference between the two treatment groups in terms of the change in creatinine values. This study indicated that treatment change to TAF and ETV was safe in terms of renal functions.
The adverse effect of TDF on BMD has been associated with phosphorus excretion and increased bone turnover (9). Previous studies demonstrated that in CHB patients, TDF was a risk factor for a T-score ≤ -1 in week 24 of treatment and a 2% decrease in hip and spinal bone density (31, 32). A study reported that the bone loss associated with TDF was observed in the hip, not the spine (32). An improvement has been shown in BMD in the early period after the transition from TDF to TAF in real-life cohorts (14). In the current study, there was a significant enhancement in the BMD rate in the TAF and ETV groups in the sixth month, and there was no significant difference between them regarding the prevalence of BMD (22). The findings of our research indicated that ETV and TAF regimens were safe for improving BMD.
TAF therapy resulted in high LDL cholesterol levels in more patients than TDF therapy (15, 19, 33). Significant reductions have been reported in triglycerides, LDL cholesterol, and HDL cholesterol levels of the CHB patients receiving TDF compared to ETV therapy (34). Although the mechanism of the lipid-lowering effect was unknown, the positive effect of TDF on lipid expression has been revealed in various studies on patients with HIV/AIDS (35). In this study, we observed increases in all lipid parameters with both TAF and ETV following the cessation of TDF therapy. The rise in LDL cholesterol was significant in both treatment groups. On the other hand, the change in lipid profile was not significantly different between the TAF and ETV groups. Our study indicated that TAF and ETV had similar impacts on the lipid profile of patients. We suggested evaluating the cardiac condition of the patients who have undergone treatment changes.
The main strength of this study was that real-life data were presented in a multicenter study from a region where hepatitis B infection is moderately endemic. To the best of our knowledge, our study is the largest series comparing treatment responses and changes in parameters with these treatment regimens. However, the use of retrospective analysis is one of the limitations of this study, and our results must be confirmed by prospective studies with larger series. Another limitation is that in this retrospective study, all evaluated parameters were not demanded, with the preference of the treating physician. This resulted in a reduction in the number of patients to evaluate each effect.
5.1. Conclusion
During the long-term treatment management of CHB, changing drug regimens to safe and effective medications may be required. In the clinical situations of TDF discontinuation, ETV and TAF are considered effective alternative treatments. Our study showed that ETV and TAF switching sustained viral suppression and biochemical response achieved by TDF therapy. The renal dysfunction and reduced BMD caused by TDF can be controlled by switching treatment to TAF or ETV.