1. Background
Although nucleos(t)ide analogs (NA) are essential in the effective management of chronic hepatitis B (CHB), still the ultimate goal of treatment, which is the hepatitis B virus (HBV) eradication (so-called sterilization cure), cannot be achieved with the available drugs. The realistic goal of treatment is to accomplish viral hepatitis B surface antigen (HBsAg) clearance with or without anti-HBs development, which is called a functional cure. Currently, the predominant form of CHB in Europe is e antigen (HBeAg) negative chronic HBV infection. In HBeAg-negative patients, monitoring treatment efficacy is difficult due to the persistent suppression of viral replication and the normal activity of liver enzymes.
Recently, the quantification of HBsAg was proposed as a helpful tool in HBeAg-negative CHB natural course monitoring and treatment control. Serum HBsAg is supposed to reflect the amount and transcriptional activity of covalently closed circular deoxyribonucleic acid (cccDNA), which is located in the nuclei of infected hepatocytes and serves as a template for all HBV messenger ribonucleic acid (RNA) and the pregenomic RNA (1). The HBsAg titer is higher in HBeAg-positive patients than in HBeAg-negative subjects, where it declines slowly during the natural progression of the disease (2).
Five NA have been registered to treat HBV infection; however, only two of them, namely entecavir (ETV) and tenofovir dipivoxil (TDF), are currently recommended as the first-line treatments due to their high potency and high resistance barrier. Nevertheless, NA have a minor effect, if any, on cccDNA. This finding means that ETV and TDF should not have any influence on the quantity of HBsAg (qHBsAg) during treatment, and the role of surface antigen measurements during NA therapy has not been fully elucidated. Some authors have shown that HBsAg levels decrease slowly during long-term treatment with potent NA. The drop below certain values might predict HBsAg loss; however, whether it is attributable to NA themselves or a spontaneous decline during the natural course of CHB remains unclear (3, 4). In other studies, no significant decline in HBsAg levels was shown in the majority of patients treated with ETV (5). There are only a few studies investigating the kinetics of HBsAg decline during TDF therapy or comparing the effect of ETV and TDF on the changes of HBsAg in CHB patients.
2. Objectives
The current study aimed to assess the effect of ETV and TDF on qHBsAg kinetics in HBeAg-negative CHB patients and estimate the time necessary to achieve HBsAg clearance with each of these drugs.
3. Methods
3.1. Study Subjects
Caucasian patients with HBeAg-negative CHB, treated for at least one year with either ETV or TDF in standard doses, were enrolled in this study. The CHB was diagnosed using serum HBsAg positivity for at least 6 months and confirmed by liver biopsy in each case. Only patients with the baseline serum HBV DNA and quantitative HBsAg measurements were considered. Most patients cleared HBV DNA after 6 months of treatment (median HBV DNA at 6 months: 0 copies/mL; range: 0 - 1610 copies/mL) and remained negative under NA throughout the study period. Every treated patient had undetectable HBV DNA by the 30th month. The patients who had less than three quantitative HB measurements during the study period were excluded. Other exclusion criteria were combination therapy with NA and interferon (IFN), virological breakthrough during NA treatment, treatment termination due to pregnancy or other medical conditions, and coinfections with other viruses (eg, hepatitis C virus, hepatitis D virus, or human immunodeficiency virus).
3.2. Laboratory Assays
3.2.1. Alanine Aminotransferase
Serum alanine aminotransferase levels were measured by an automated analyzer (COBAS 8000, Roche Diagnostics, Germany). The upper limit of normal was set at 33 U/L for females and 42 U/L for males.
3.2.2. Serological Assays
Serum HBsAg, HBs antibodies, HBeAg, and HBe antibodies were detected by commercially available immunoassays (ECLIA, COBAS 8000 analyzer, Roche Diagnostics, Germany). The measurements of serum HBsAg titers were performed with the Elecsys HBsAg II Quantitative assay (Roche Diagnostics, Germany) with the dynamic range of 0.05 - 130 IU/mL and automatic onboard dilution, increasing the upper limit of detection to 117 000 IU/mL.
3.2.3. Molecular Assays
The HBV DNA was measured by real-time polymerase chain reaction using Cobas Taqman assay (Roche Diagnostics, Germany) with a linear range of 10 - 1,000,000,000 IU/mL.
3.3. Statistical Analysis
Categorical data are expressed as numbers (percentages). Continuous variables are described as means with standard deviations or medians with their interquartile range (IQR). The relationship between quantitative HBs and other variables was assessed using Spearman’s rank correlation coefficient. The chi-square and Mann-Whitney U test or student’s t-test were used for comparisons between different treatment groups. Paired data were assessed using the Wilcoxon signed-rank test. The estimated time to undetectable HBsAg was calculated using the best-fitted curve analysis. A P-value of less than 0.05 was considered statistically significant. All the calculations were performed using Statistica 13.1 software (StatSoft, Kraków, Poland).
4. Results
The study group comprised 93 patients (58 male and 35 female subjects) with a mean age of 48.6 ± 13.8 years. The median follow-up duration was 42 months, and the median number of HBsAg examinations was eight measurements. Table 1 shows the clinical characteristics of the patients.
| Study Parameter | All | TDF | ETV | P-Value |
|---|---|---|---|---|
| Number of patients | 93 | 39 | 54 | |
| Age (y) | 48.6 ± 13.8 (26 - 88) | 45.1 ± 13.7 (26 - 78) | 51.1 ± 13.5 (26 - 88) | 0.08 |
| Male gender | 58 (62.4) | 26 (66.7) | 32 (59.3) | 0.548 |
| Fibrosis | 1 | 1 | 1 | 0.75 |
| F0 | 10 (16.1) | 3 (13) | 7 (17.9) | 0.75 |
| F1 | 30 (48.4) | 12 (52.2) | 18 (46.2) | 0.696 |
| F2 | 7 (11.3) | 2 (8.7) | 5 (12.8) | 0.795 |
| F3 | 2 (3.2) | 0 | 2 (5.1) | 0.739 |
| F4 | 13 (21) | 6 (26.1) | 7 (17.9) | 0.603 |
| G0 | 6 (10.7) | 2 (9.1) | 4 (11.8) | 0.974 |
| G1 | 34 (60.7) | 15 (68.2) | 19 (55.9) | 0.448 |
| G2 | 12 (21.4) | 4 (18.2) | 8 (23.5) | 0.746 |
| G3 | 3 (5.4) | 0 | 3 (8.8) | 0.589 |
| G4 | 1 (1.8) | 1 (4.5) | 0 | 0.784 |
| ALT (U/L) | 51.0 ± 65.9 (12 - 440) | 61.8 ± 72.3 (14 - 389) | 43.2 ± 60.3 (12 - 440) | 0.103 |
| HBV DNA (copies/mL) | 846 (0 - 9729) | 3240 (38 - 84800) | 32.5 (0 - 6050) | 0.004 |
| HBsAg (IU/mL) | 8731 (2173 - 18807) | 7727 (3338 - 18807) | 9400 (1661 - 20770) | 0.849 |
| Follow-up (mon) | 42 (24 - 48) | 36 (24 - 48) | 48 (42 - 48) | 0.000 |
| Number of HBsAg measurements | 8 (4 - 9) | 4 (4 - 8) | 9 (8 - 9) | 0.000 |
Abbreviations: TDF, tenofovir dipivoxil; ETV, entecavir; ALT, alanine aminotransferase; HBV, hepatitis B virus; DNA, deoxyribonucleic acid; IQR, interquartile range; HBsAg, hepatitis B surface antigen
a All the continuous data were represented by the mean + SD (for normally distributed data) or by the median and interquartile (for non-normally distributed data). Categorical data were presented by the No. (%).
4.1. Baseline
In this study, 54 (58.1%) and 39 (41.9%) patients were treated with ETV and TDF, as shown in Table 2, respectively. There were no statistically significant differences in age and gender distribution between the treatment groups (P = 0.08 and P = 0.548, respectively) and in the grade and stage of liver disease (P ≥ 0.448). The median quantitative HBV DNA values for ETV and TDF were 9400 U/mL and 7727 U/mL at the baseline, respectively. The aforementioned values did not differ significantly (P = 0.849). Significantly higher HBV DNA levels at the baseline were observed in the TDF group (median: 3240 IU/mL; IQR: 38 - 84800) than in EDV-treated patients (median: 32.5 IU/mL; IQR: 0-6050) (P = 0.004).
| HBsAg (IU/mL) | All | TDF | ETV | P-Value | |||
|---|---|---|---|---|---|---|---|
| Value | Range | Value | Range | Value | Range | ||
| Baseline | 8371 | 2173 - 18807 | 7727 | 3338 - 18807 | 9400 | 1661 - 20770 | 0.849 |
| 6 months | 7659 | 1824 - 18875 | 6098.5 | 1917 - 13160 | 8541 | 1730 - 20670 | 0.944 |
| 12 months | 6966 | 1593 - 16480 | 5617 | 3671 - 15815 | 7072 | 1274 - 18480 | 0.944 |
| 18 months | 6349 | 1164 - 17422 | 4782 | 1375 - 13862 | 6720 | 1076.7 - 20840 | 0.735 |
| 24 months | 6631 | 1460 - 19320 | 5859 | 3791 - 15836 | 8434 | 1167 - 19688 | 0.964 |
| 30 months | 6094 | 803.1 - 16280 | 5230 | 810.2 - 13980 | 6135 | 711.1 - 16920 | 0.979 |
| 36 months | 5831 | 1324 - 17830 | 5705 | 2879 - 17830 | 5893 | 844.5 - 17120 | 0.791 |
| 42 months | 4715 | 640.7 - 12920 | 3515 | 2270 - 16980 | 5484 | 637.4 - 12667 | 0.842 |
| 48 months | 4488.5 | 934.2 - 16000 | 3572.5 | 2290 - 18490 | 5428 | 645.7 - 15750 | 0.393 |
| Patients with HBsAg drop, No. (%) | 79 (84.5) | 34 (87.2) | 45 (83.3) | 0.754 | |||
| Drop rate (IU/mL per month) | 120.4 ± 158.1 | 0.008 - 720.7 | 145.6 ± 152.17 | 0.009 - 720.7 | 101.4 ± 117.5 | 0.16 - 478.3 | 0.362 |
| Overall HBsAg drop (IU/mL) | 2003 | 638.1 - 5010 | 2286 | 730 - 4565 | 1808 | 519.7 - 6810 | 0.84 |
| Overall HBsAg drop (% vs. baseline) | 40.3 ± 25.9 | 1.2 - 100 | 40.4 ± 55.9 | 1.7 - 100 | 40.3 ± 26.4 | 1.3 - 98.7 | 0.887 |
Abbreviations: TDF, tenofovir dipivoxil; ETV, entecavir; IQR, interquartile range; HBsAg, hepatitis B surface antigen
a Values are expressed as median, IQR unless otherwise indicated.
4.2. Correlation Between Serum HBsAg and Other Parameters
There was a negative correlation between HBsAg titer at the baseline with patients’ age (rho = -0.47; P = 0.000), inflammation (rho = -0.28; P = 0.038), and fibrosis grade (rho = -0.33; P = 0.009) in liver biopsy. No significant correlation was observed between quantitative HBsAg and HBV DNA levels at the baseline (P = 0.242).
4.3. 48 Months
The observation time was shorter for TDF than for the ETV group, with a median of 36 and 48 months, respectively (P = 0.000). Fewer samples were taken in the TDF group (P = 0.000). Overall there was a decrease in serum quantitative HB at 48 months in 79 patients (84.9%). This trend was apparent in 34 (87.2%) TDF-treated patients and 45 (83.3%) ETV-treated patients (P = 0.754).
The median quantitative HBsAg drop was 2003 IU/mL (IQR: 638.1 - 5010), and there were no statistically significant differences between the study groups. The HBsAg levels decreased by 40.3 ± 25.9% on average. The mean HBsAg drop rate was 120.4 ± 158.1 IU per month. In this study, a single case of HBsAg loss with subsequent anti-HB development was detected. This clinical achievement was observed when the patient was under TDF therapy. The HBsAg levels were significantly lower at 12, 24, and 48 months in both treatment groups than the baseline (P ≤ 0.022). At 48 months, the median HBsAg levels were 3572.5 (IQR: 2290 - 18490 IU/mL) and 5428 (IQR: 645.7 - 15750 IU/mL) for TDF and ETV, respectively (P = 0.393).
4.4. Estimated Time to Undetectable HBsAg
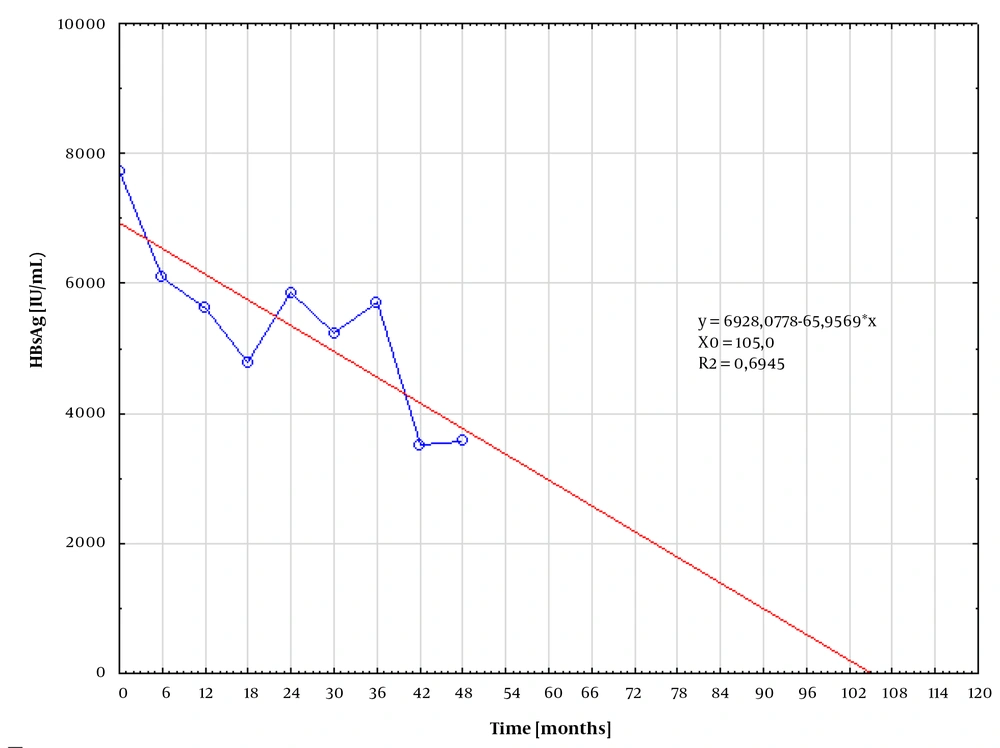
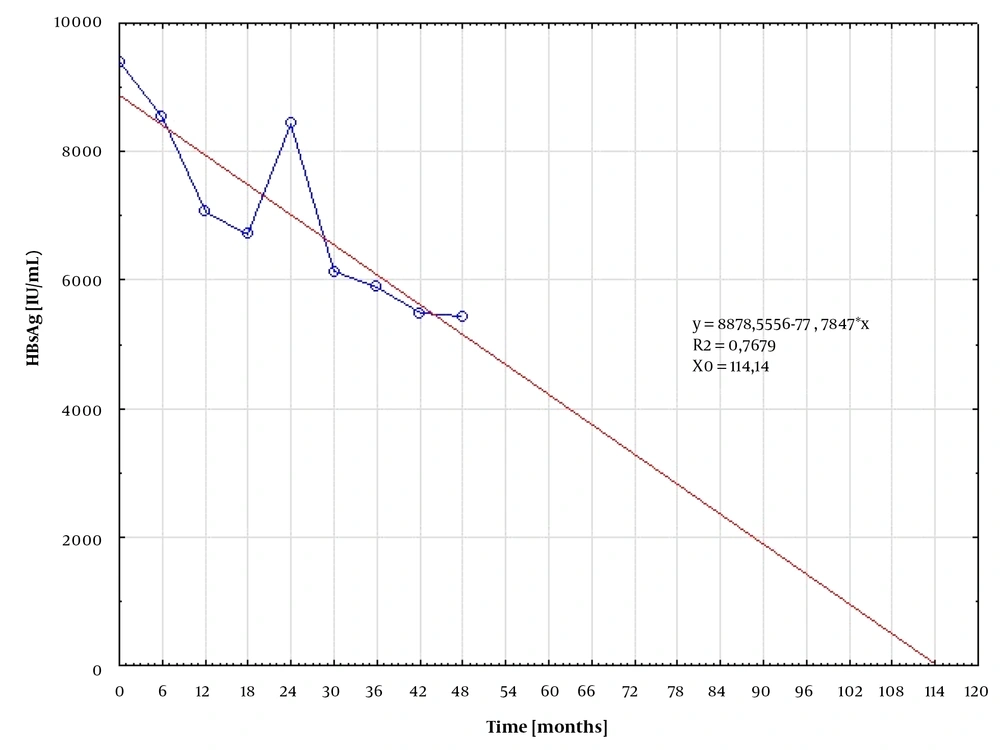
In patients responding to treatment, the median quantitative HB levels were plotted against time, along with the best-fitted curve. The time to undetectable HBsAg was subsequently calculated from the resulting equation. The calculated time for HBsAg loss was 105.0 and 114.14 months for patients treated with TDV (Figure 1) and ETV (Figure 2), respectively.
5. Discussion
The maintenance of viral suppression is the main goal of anti-HBV therapy; therefore, HBV DNA measurements play a key role in the determination of NA treatment efficacy. However, in patients with persistent viral suppression and repeatedly normal aminotransferases long-term treatment control, the identification of possible therapeutic stop points is difficult. The only recommended end-point to stop NA therapy in HBeAg-negative patients is HBsAg loss; nevertheless, it is rarely achieved (6).
Quantitative HBsAg detection became a helpful tool to monitor NA therapy because it was noticed that a significant decline in HBsAg levels during therapy might predict later HBsAg loss or allow to decide on treatment termination. Retrospective studies suggest that HBsAg drop below 100 IU/mL predicts the long-term sustained immune control of HBV infection and justifies an attempt to discontinue NA therapy (7). On the other hand, the interpretation of reduction in HBsAg levels in e negative patients treated with NA and its relation to intrahepatic cccDNA content is burdened with several pitfalls. Viral minichromosome is not the only source of HBsAg. In comparison to HBeAg-positive patients, in HBeAg-negative subjects, the amounts of HBV DNA integrated with the host genome, also producing HBsAg, but not the complete viral genome, are higher and might significantly influence HBsAg titers (8). The other reasons for inaccurate quantitative HBsAg results are the possible formation of immune complexes with anti-HBs antibodies and the development of HBsAg escape mutants (9).
Nevertheless, HBsAg quantification, albeit not fully accurate, is currently the only widely available tool reflecting cccDNA transcriptional activity and content. According to the results of the present study, quantitative qHBsAg steadily declined under NA treatment in approximately 85% of patients who achieved undetectable HBV DNA. That was not related to the baseline HBV DNA level. Similar results were obtained by Seto et al. (4). The declining trend was comparable in ETV- and TDF-treated patients, although the TDF group was smaller and the mean observation time was shorter. In addition, the average number of quantitative HB results per individual was smaller in the TDF group. The reason for these statistically significant differences was the later introduction of TDF into the national HBV treatment program. The median reduction in quantitative HBsAg was 2000 IU/mL within at least one year of treatment, and this decline was similar in ETV- and TDF-treated patients. It means that the average HBsAg level dropped by 40 ± 26% in the analyzed period.
In this study, it was not managed to confirm that the inhibitory effect against HBV was stronger on TDF than on ETV, as suggested in previous studies (10). Using the best-fitted curve analysis, the expected time required for HBsAg clearance was comparable in both groups, equaling 104 and 114 months for TDF and ETV, respectively. This finding suggests that in order to clear HBsAg, the therapy should be continued for an additional 5 years. Berg et al. found that 43% of HBeAg-negative patients who stopped treatment with TDF after at least 3.5 years (42 weeks) with suppressed HBV DNA achieved either HBsAg loss or sustained immune control by the 144th week (11).
In another study on HBsAg kinetics under different NA, it was shown that the estimated time required to clear HBsAg was the shortest, calculated for 17.2 years, in patients treated with TDF as compared to another NA (10). Li et al. studied HBsAg decline in patients who underwent 6-year monotherapy with ETV and concluded that the treatment duration necessary for HBsAg clearance was 25 years (12). According to the results of Zoutendijk et al.’s study (3), it needs approximately four decades for HBsAg loss either for ETV or TDF. However, in the present study, HBsAg decline was observed for a relatively short period (2 years).
5.1. Conclusions
In conclusion, serum quantitative HBsAg gradually decreased on ETV and TDF treatment in most patients who achieved persistent viral suppression. If such a declining trend continues steadily during long-term treatment with NA, a proportion of patients might achieve HBsAg loss approximately after a decade. The potency of ETV and TDF in HBsAg reduction seems to be similar.