1. Background
Liver transplantation is the treatment of choice for those with end-stage liver disease (1, 2). Due to immunosuppressant therapy that is required to minimize the likelihood of graft rejection, recipients are at a high risk of opportunistic infections, such as reactivation of latent viral infections.
Cytomegalovirus (CMV) and human herpes virus-6 (HHV-6) are both viruses from the β-herpesviridae family that are very common following transplantation. People around the globe are usually exposed to these viruses early in life and they establish latent infections, which last during their lifetime with potential reactivations later (3-6). Epidemiological studies have shown seropositivity to CMV and HHV-6 in the adult population to be 75% to 100% (7-9) and 80% to 90% (10, 11), respectively. These herpes viruses have immunomodulatory effects that can up-regulate alloantigens, hence can enhance the risk of acute allograft rejection as well as chronic graft injury (12). They are purported to have a considerable role in post-transplantation morbidity and mortality (13-15).
Recent studies have shown that the reactivation of HHV-6 can predispose liver transplant recipients for CMV infection (16-18), and suggested that HHV-6 reactivation precedes CMV infection in time (19, 20). There is a paucity of information regarding the time of HHV-6 and CMV activation after transplantation. This study aimed at investigating the development and timing of CMV infection after liver transplantation and its relation to post-transplantation HHV-6 activation.
2. Methods
All patients undergoing liver transplantation from April to August 2009 were enrolled in this study, regardless of their age, place of residence, or their liver failure etiology. This time of the year, i.e. late spring-early summer was chosen deliberately to avoid confounders, like seasonal viral infections, on patients’ clinical manifestation. Informed consent was obtained from all participants. The study was approved by the institutional review board of Shiraz University of Medical Sciences, Shiraz, Iran, and it was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Transplantation-related treatments: All transplant recipients received immunosuppressive therapy with Tacrolimus, Mycophenolate mofetyl, and corticosteroids. In case of increased bilirubin, Tacrolimus was replaced with cyclosporine. In case of acute rejection, a 3-day pulse-therapy with methylprednisolone was administered. Acute allograft rejection was diagnosed and determined using the Banff international consensus criteria (21). Anti-viral prophylaxis was done routinely at the researcher’s institution. Patients with post-transplantation clinical presentation of CMV infection were treated with intravenous Ganciclovir 10 mg/kg/day in 2 divided doses for 2 to 3 weeks.
Blood sampling: Ten milliliters of blood were collected from each recipient at baseline (1 day before transplantation) and every week after that for a period of 12 weeks. There was a median of 12 samples per patient. Given that patients in this study were under treatment with immunomodulating drug regimens that could cause leukopenia and may consequently affect the validity of CMV antigenemia, it was decided to draw 10 mL of oxalated blood from each patient to be able to produce pre-specified counts of PMN. Post-transplantation blood sampling occurred either at Namazi Hospital of Shiraz in case of hospitalization, or as an outpatient at Prof. Alborzi clinical microbiology research center, Shiraz, Iran. For patients, who were not a resident of Shiraz and had returned to their hometown after being discharged from the hospital after transplantation, the blood specimens were taken at a public hospital of the city and transported to Shiraz, and maintained in the cold chain. The ethylene-diamine-tetra-acetic acid (EDTA)-treated blood samples were centrifuged and the sera and leukocytes were aliquoted and frozen at -70°C until further testing for CMV and HHV-6.
ELISA tests: For detecting the anti-CMV IgG seropositivity, the enzyme-linked immunosorbent assay (ELISA) kit of Genesis Diagnostics Ltd., UK was used. IgG antibodies to HHV-6 were detected by an ELISA kit from Biotrin International Ltd., Dublin, Ireland. Tests were done according to manufacturers’ instructions, also as previously described by Behzad-Behbahani et al. (22)
CMV pp65 antigenemia: To determine post-transplantation viral activation of CMV, the EDTA-treated blood samples for CMV antigenemia was assessed, by evaluating the presence of lower matrix pp65 antigen in polymorph nuclear cells using the CMV Brite Turbo Kit (IQ products, Groningen, Netherlands), performed according to manufacturer’s instruction, as described previously by Saadi et al. (23).
DNA extraction: Genomic DNA was extracted from buffy-coated EDTA-treated samples using a QIAamp DNA mini Kit (Qiagen, Germany), according to manufacturer’s instructions (24). The extracted DNA was processed in a search for HHV-6 and CMV DNA, using the polymerase chain reaction (PCR) technique.
CMV and HHV-6 PCR assays: Using a specific oligonucleotide primer set from a major immediate early gene of CMV and major capsid protein gene of HHV-6, a thermocycler PCR assay was conducted to amplify the genomes in the serum and leukocyte specimens. The HHV-6A and HHV-6B variants were also identified. The primers, which were used for the first and second rounds of the CMV PCR had a nucleotide sequence of 5′-GTCTACGGATTGCTGACGCT-3' and 5′-TTGCAGGCCACGAAC GT-3′ for outer pairs and 5′-ACCGCTTTCAGCGTACTCAT-3′ and 5′-ACATACAGCG CAAAC ACCAG-3′ for inner pairs, respectively. The inner primers amplify a 179-bp fragment of CMV immediate early gene. The primers that were used for the first and second round of the HHV-6 PCR assay had a nucleotide sequence of 5'-GCTAGAACGTATTTGCTG-3' and 5'-ACAACTGTCTGACTGGCA-3' for outer pairs and a sequence of 5'- TCACGCACATCGGTATAT-3' and 5'- CTCAAGATCAA CAAGTTG-3' for inner pairs; the inner pairs amplified a 167-bp fragment of HHV-6 gene. The PCR samples consisted of 5 μL extracted DNA with 0.2 mM of dNTP, 1.5 mM MgCl2, 2U Taq DNA polymerase (Fermentas, Lithuania), and 0.5 μM of each specific primer with PCR reaction buffer (Fermentas, Lithuania), summed up to a final volume of 50 μL. For positive control, the plasmid DNA was used and for negative controls, CMV and HHV-6 negative DNA were included as well as no template control in each experiment.
The first round of CMV-PCR was conducted at 94°C for 3 minutes, which was followed respectively by 30 cycles of 94°C for 40 seconds, 61°C for 40 seconds, and 72°C for 40 seconds. After that, a terminal extension of 72°C was conducted for 5 minutes. The product of the first round was used as a template for the second round, which was carried out with the same conditions described for the first round. The first round of HHV-6 PCR was performed at 94°C for 3 minutes, followed, respectively, by 30 cycles of 94°C for 40 seconds, 51°C for 1 minute, and 72°C for 40 seconds. This was followed by a terminal extension of 72°C for 5 minutes. The product of the first round was used as a template for the second round, which was carried out with the same conditions that were described for the first round. Eventually, conventional gel electrophoresis and ethidium bromide staining were used to detect the amplified products and to analyze the PCR products (22). The PCR assay that was used for detecting CMV DNA in this study had a sensitivity of 100% for detecting 10 copies of the target sequence per microliter of the extracted DNA, thus 10 copies/µL was considered as the threshold for positive CMV PCR.
Statistical analysis: Categorical variables were presented as frequency (percentage) and continuous variables were presented as mean ± standard deviation or median (range). The independent t-test was used to compare continuous variables between groups with and without symptomatic CMV infection, and chi-squared test or Fisher’s Exact test to compare categorical variables between these two groups. To explore the alignment of results from different laboratory tests, the Pearson correlation coefficient was used. All analyses were done using the SPSS software version 21.0 (Chicago, IL). P values of ≤ 0.05 were considered statistically significant.
3. Results
A total of 46 liver transplant recipients were enrolled in this study, 25 (54.3%) of which were male. None of the recipients were symptomatic for CMV or HHV6 infection at baseline. All patients were followed for a median period of 3 months. Seventeen (36.9%) developed clinical symptoms related to CMV infection during the follow-up period after transplantation, which were presented as hepatitis (n = 6), colitis (n = 5), pneumonitis (n = 4), retinitis (n = 1), and meningoencephalitis (n = 1). Amongst the symptomatic patients, 8 (47.1%) were male and 9 (52.9%) were female (P = 0.21). Median age was 22 years old with a range from 1 to 62 years old. The most frequent age group was the 26- to 30-year-old group with 10 (21.7%) patients. The frequency of patients in each age group, stratified with the presence of symptomatic CMV infection, is depicted in the supplementary file appendix 1.
Among 46 patients, 42 (91.3%) were seropositive for CMV before transplantation. All 4 pre-transplant seronegative cases (100.0%) became symptomatic for CMV infection post-transplantation during the follow-up period, whilst in the pre-transplant seropositive group, only 13 (30.9%) became symptomatic for CMV infection after transplantation.
Among 46 participants, 41 (89.1%) were seropositive for HHV-6 at baseline. There was a statistically significant relationship between pre-transplant CMV and HHV-6 seropositivity and age (P = 0.013 and 0.015, respectively).
Twenty-three patients (50.0%) were positive in terms of antigenemia for CMV, among which 17 (73.9%) became symptomatic for CMV infection. The minimum and maximum antigenemia in the symptomatic group was 7 to 72 per 200,000 PMN, whilst the rate was 0 to 12 per 200,000 PMN in the asymptomatic group (P = 0.001).
As demonstrated in Table 1, the viral load of CMV in the serum was significantly higher in symptomatic patients as compared to the asymptomatic group (P = 0.008). Half of the patients (50.0%) had positive results for both sera and leukocytes for CMV PCR. There was a significant relationship between the CMV viral load in serum and leukocytes and the development of clinical symptoms for CMV infection (both P = 0.001).
| Total (n = 46) | Symptomatic (n = 17) | Non-Symptomatic (n = 29) | |
|---|---|---|---|
| Age | 25.2 ± 3.9 | 17.4 ± 3.5 | 23.9 ± 6.5 |
| Male sex | 25 (54.3) | 8 (47.1) | 17 (58.6) |
| Baseline | |||
| CMV seropositivity | 42 (91.3) | 13 (76.4) | 29 (100.0) |
| HHV-6 seropositivity | 41 (89.1) | 13 (76.4) | 28 (96.5) |
| Post-Tx | |||
| Positive CMV antigenemia | 23 (50.0) | 17 (100.0) | 6 (20.6) |
| Mean pp65 antigenemia (per 200,000 PMN) | 22.46 ± 1.32 | 42.4 ± 5.4 | 1. 5 ± 0.6 |
| Median pp65 antigenemia (per 200,000 PMN) | 12.2 | 15.1 | 5.1 |
| CMV viremia (serum PCR) | 23 (50.0) | 17 (100.0) | 6 (20.6) |
| Mean CMV viral load in serum (copies/mL) | 5.082.0 ± 341.0 | 12.064.6 ± 1.590.4 | 215.7 ± 82.5 |
| Median (min-max) | 8.521 (0 - 6.720) | 11.609 (501 - 24.309) | 119 (0 - 2.971) |
| CMV viremia (leukocytes PCR) | 23 (50.0) | 56.739 ± 7.792.2 | 2.630 ± 1.195.5 |
| Mean CMV viral load in PMNs (copies/mL) | 27.375 ± 3.245 | 56.735 ± 7.797 | 2.630 ± 1.195 |
| Median (min-max) | 38,453 (0 - 58.561) | 50.568 (6.850 - 120.890) | 274 (0 - 28.064) |
| Positive HHV-6 PCR | 25 (54.3) | 14 (60.9) | 9 (39.1) |
| Mean HHV-6 viral load; copies/mL | 7.634 ± 481 | 11.283 ± 2.326 | 1.776 ± 680 |
| Median (min-max) | 7.636 (0 - 36.527) | 9.806 (0 - 36.527) | 0 (0 - 14.087) |
| Concurrent CMV and HHV-6 PCR positivity | 41 (89.1) | 13 (76.5) | 4 (23.5) |
Abbreviations: CMV, cytomegalovirus; HHV-6: human herpes virus 6; PCR, polymerase chain reaction; PMN, poly morph nuclear cells; Tx: transplantation.
aValues are expressed as mean ± SD or No. (%).
There was a significant correlation between CMV antigenemia and PCR results in serum (P = 0.003) and PMNs (P = 0.001) in the current study (Table 2). On the other hand, CMV viral load in the serum was correlated with CMV viral load in leukocytes (P = 0.001). The CMV activation defined by PCR and pp65 antigenemia were not correlated with patients’ age (P = 0.35).
| Symptomatic CMV | pp65 Antigenemia | Serum CMV PCR | Leukocyte CMV PCR | |
|---|---|---|---|---|
| Symptomatic CMV | 1 | 0.001 | 0.008 | 0.001 |
| pp65 antigenemia | 0.001 | 1 | 0.003 | 0.001 |
| Serum CMV PCR | 0.008 | 0.003 | 1 | 0.001 |
| Leukocyte CMV PCR | 0.001 | 0.001 | 0.001 | 1 |
Abbreviations: CMV, cytomegalovirus; PCR, polymerase chain reaction.
Twenty-five (54.3%) patients were positive for HHV-6 PCR. The HHV-6 viral load was higher in patients with symptomatic CMV infection compared to those without a symptomatic CMV infection. There was a significant relationship between positive results for HHV-6 and the development of clinical presentation (P = 0.002), yet there was no statistically-significant association between pre-transplantation seropositivity of HHV-6 and the subsequent CMV infection after transplantation (P = 0.055). The rate of HHV-6 PCR positivity was higher in those with positive CMV PCR as compared to those with negative CMV PCR results (60.9% vs 47.8%), yet the chi-squared analysis showed no significant relationship between the positivity of these two viruses in PCR (P = 0.27) (Table 3).
| CMV | |||
|---|---|---|---|
| Positive | Negative | ||
| HHV-6 | Positive | 14 (60.9) | 11 (47.8) |
| Negative | 9 (39.1) | 12 (52.2) | |
Abbreviations: CMV, cytomegalovirus; HHV-6, human herpes virus 6.
Among 25 patients, who were positive for HHV-6, 22 had the B variant of HHV-6 and only 3 were of the A variant, all of which were symptomatic for CMV infection after transplantation. The HHV-6 viral load was higher in patients with variant A compared to those with variant B (11631 ± 3162 vs 9478 ± 2767), yet the difference was not statistically significant (P = 0.42). Also, there was no relationship between the HHV-6 variants and the development of symptomatic CMV infection after transplantation (P = 0.53).
The average post-transplantation time for becoming positive for CMV was 26.3 ± 17.9 days in the group with symptomatic CMV infection and 8.5 ± 3.6 days in the asymptomatic group. Among different diagnostic methods, the detection of viral DNA in leukocytes by PCR was the fastest way, as compared to the PCR of serum samples, pp65 antigenemia, or serologic studies.
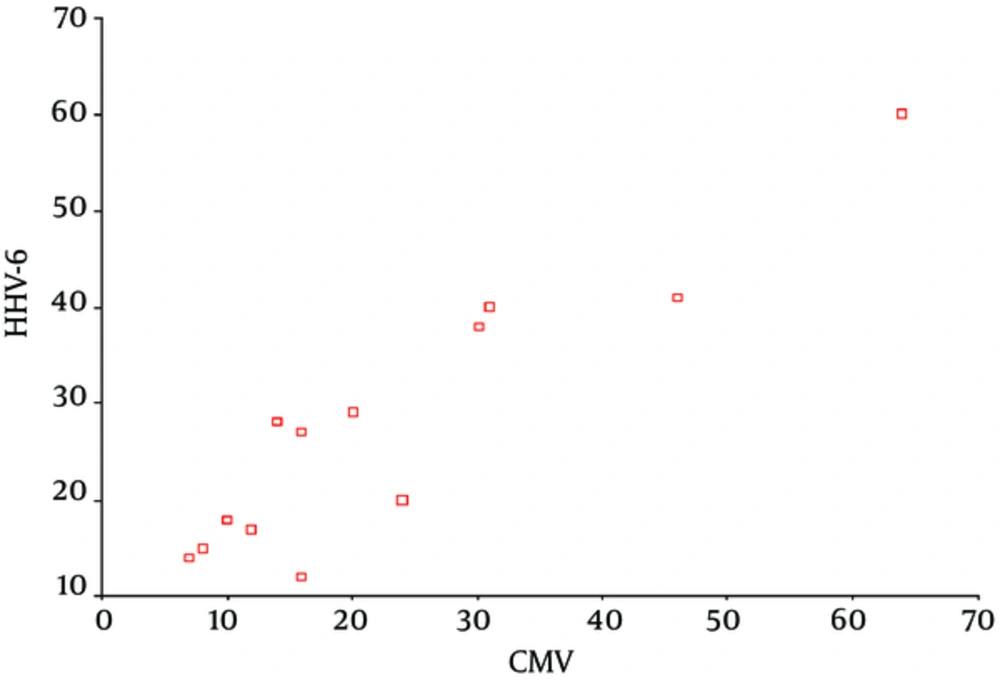
The average post-transplantation time for activation of HHV-6 was 17.5 ± 4.2 days in the group with symptomatic CMV infection and 8.6 ± 2.1 days in the asymptomatic group. Among 13 symptomatic patients, who were found to be positive for both CMV and HHV-6, HHV-6 was activated earlier than CMV in 10 patients and the average time for HHV-6 activation was 19.8 ± 4.5 days whilst it was 28.4 ± 60.5 days for CMV activation (P = 0.001), which showed a 9-day time gap between the activation of these two viruses in liver transplant recipients. The regression analysis of the times of post-transplantation activation of CMV and HHV-6 showed a linear relationship between the activation time of these two viruses (Figure 1).
All symptomatic patients were treated with Ganciclovir. Two symptomatic patients deceased during the follow-up period. One case was a 62-year-old patient, who developed CMV infection and hepatitis after transplantation and died despite the anti-viral treatment. Another patient had an age of < 5 years and died after a concurrent CMV and HHV-6B infection. Also, an adult patient developed neurologic symptoms, i.e. epilepsy, yet survived after being infected with CMV and the A-variant of HHV-6, which is known to be more neurotropic. The remaining patients responded well to anti-viral treatment. Clinical symptoms were alleviated and the viral loads went back to zero after treatment.
4. Discussion
It was shown that HHV-6 activation is associated with CMV activation and the development of symptomatic disease. In terms of timing of activation, HHV-6 activation precedes the detection of CMV DNA in serum and leukocyte samples. Considering that in this study all liver transplant recipients were included irrespective of their age, place of residence or liver failure etiology, this study was more inclusive and seems to be representative of the target population compared to previous literature (16, 25, 26).
As the most common age group for symptomatic disease in the follow-up period was the group with 1 to 5 years of age (23.5%), the lack of previous exposure and negative baseline serology in this age group may be a potential factor. Nevertheless, chi-squared analysis showed no significant relationship between age groups and the development of symptomatic disease (P > 0.05).
In the study of Saghafi et al. all donors (n = 925) and recipients (n = 710) were shown to be seropositive for CMV prior to transplantation (27). In the current study, 91.3% of cases were seropositive for CMV at baseline. The slight difference in the seropositivity of recipients between studies could be attributed to age differences, as the afore-mentioned study only included adults, whilst the patients in this study were from a broad range of age. Seronegative recipients were at a higher risk of symptomatic infection compared to CMV IgG seropositive ones, which confirms the findings of previous studies (26, 28-30).
The studies that investigated CMV and HHV-6 activation in transplant recipients generally have not studied the pre-transplantation serology of HHV-6 (16, 25, 26, 31, 32). In this study, 89.1% of recipients were seropositive for HHV-6 at baseline. However, there was no significant relationship between HHV-6 seropositivity and post-transplantation symptomatic CMV infection.
The occurrence of post-transplantation CMV infection varies among studies, which could be due to different study populations, different diagnostic criteria (25), longer follow-ups (20), the use of anti-viral prophylaxis in D+/R- transplantations (16, 26), etc.
As expected, the pp65 antigenemia results were in accordance with the PCR results of both serum and leukocyte extracts. No antigenemia-positive patient was identified as negative using the PCR method. Detection of CMV was faster using the PCR technique as compared to the pp65 antigenemia method (7 days earlier detection on average). These findings were consistent with previous studies (33). Based on the CMV PCR results, the viral load was higher in PMN extracts as compared to serum extracts. The reason for this phenomenon could be that CMV initially involves the leukocytes and then it expands to sera.
The rate of HHV-6 viremia was 54.3% in the current study, which was similar to the findings of Harma et al. in a similar population (26, 32). Lautenschlager et al. reported concurrent HHV-6 and CMV infection in 50% of the symptomatic patients (20), whilst the rate was 60.9% (14 out of 23) in the current study. The difference could be attributed to a lower baseline HHV-6 seropositivity in that particular study.
The study of Harma et al. demonstrated the median time to CMV and HHV-6 activation to be 30 days and 9 days, respectively (25). Similarly, in the current study and a number of previous studies (20, 26, 32), HHV-6 activation is shown to occur earlier than CMV activation. Dockrell et al. showed HHV-6 seroconversion to be a predictor of CMV disease after transplantation (16). Based on the regression analysis of the times of post-transplantation activation of CMV and HHV-6, a linear relationship between the activation time of these two viruses was observed. Based on these results, the time of CMV activation could be predicted based on the HHV-6 activation time.
HHV-6 is shown to have immunomodulatory effects that could predispose patients to opportunistic infections (7). It could also be an indicator of over-immunosuppressed condition, which could itself lead to CMV reactivation/infection (34).
Several limitations are important to note. First, most liver grafts were donated after brain death in our setting and most organs were being transported to Shiraz from other cities of Iran. Hence, it was not feasible to test the seropositivity of CMV and HHV-6 in all organ donors. Second, patients, who were not a resident of Shiraz returned to their hometowns after being discharged from the hospital on the 6th week after transplantation. Blood samples were drawn and transported to Shiraz, maintained in the cold chain, however, this precluded the use of transported samples for pp65 antigenemia testing, as this test is more time-sensitive.
In conclusion, this study showed that HHV-6 infection, either primary infection or reactivation, leads to an increased risk of CMV infection and symptomatic disease. It was also revealed that HHV-6 activation precedes the CMV activation in time and could be used as a predictor of post-transplantation CMV infection, or as a potential criterion for considering pre-emptive anti-viral therapy.