1. Context
Liver cancer is one of the most common malignancies and ranks sixth in incidence and third in mortality worldwide (1). Hepatocellular carcinoma (HCC) accounts for 75% - 85% of primary liver cancer cases. The leading causes of HCC include chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infections, heavy alcohol intake, metabolic syndrome, and aflatoxin-contaminated foodstuff (2-4). Chronic HBV infection and aflatoxin contamination are likely the predominant causes in high-risk HCC areas, such as China and sub-Saharan Africa, whereas the key determinant of HCC is chronic HCV infection in low-risk areas, such as Japan and southern and eastern Europe (1, 5). In recent years, rising obesity prevalence has contributed to the incidence of HCC in low-risk HCC areas (6, 7). Although treatments such as immune checkpoint inhibitors and anti-angiogenic targeted drugs have advanced vigorously in the past decade, the 5-year overall survival (OS) of patients with HCC is still lower than expected.
Hepatic resection is the leading treatment for early-stage HCC, while the high recurrence and metastasis rates after hepatectomy are still perplexing. Compared to postoperative prognosis, the results were controversial among patients with different hepatitis virus statuses in HCC. According to a study by Li et al. (8) on 413 patients, those with HCV-related HCC (HCV-HCC) had a lower 5-year survival rate accompanied by higher intrahepatic and multiple recurrence rates than those with HBV-related HCC (HBV-HCC) and non-HBV non-HCV HCC (NBNC-HCC) patients. However, a retrospective study found no difference in long-term outcomes (15 years) after hepatectomy between patients with NBNC-HCC and hepatitis virus HCC (666 patients) (9). Zhou et al. (10) reported that the 5-year disease-free survival (DFS) of patients with HBV/HCV-related HCC was lower than that of patients with NBNC-HCC. This study indicated a tendency toward the highest 5-year OS in the NBNC-HCC group. However, the differences were not statistically significant. There was no significant difference in the 5-year OS and DFS between the HBV-HCC and HCV-HCC groups (10).
2. Objectives
Meta-analysis is a rigorous and effective statistical tool that makes qualitative and quantitative evaluations of published scientific evidence to integrate the results better. Owing to the lack of recent, large-sample, multicenter research in the previous meta-analyses, this study aimed to compare the postoperative prognosis of HBV-HCC, HCV-HCC, and NBNC-HCC.
3. Methods
3.1. Data Sources
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (11). Two independent reviewers performed screening and data extraction, and a third reviewer resolved the differences. A number of electronic databases, including PubMed, Embase, Cochrane, Web of Science, and Scopus, were systematically searched to identify all available articles published since April 15, 2022. The keywords or medical subject headings for reference were “hepatocellular carcinoma", “hepatitis B virus", “hepatitis C virus", “survival rate", and “hepatectomy". Only human studies published in English were included.
3.2. Inclusion Criteria
The inclusion criteria for the studies were (1) cohort study design; (2) subjects being patients with HCC who underwent radical resection confirmed by postoperative pathology; (3) study outcomes of 5-year survival data that could be obtained from the article or the survival curve; and (4) all patients being serologically tested for hepatitis virus prior to surgery.
3.3. Exclusion Criteria
The exclusion criteria entailed (1) being published multiple times; (2) lacking full text and the corresponding author’s response not received; (3) incomplete data and where data were extracted effectively; (4) patients undergoing palliative care; and (5) small sample size (sample size < 30) or a large amount of censored data (lost to follow-up rate > 10%).
3.4. Data Extraction and Evidence Evaluation
Data were extracted from the texts, tables, and figures of the selected studies. The following data were extracted from each study: Publication year, first author, the number of patients, population characteristics, preoperative serological indicators, operation-related indicators, preoperative viral status, inclusion and exclusion criteria, 5-year OS, and recurrence-free survival (RFS). When 5-year OS or RFS was not directly provided in the literature, the survival curve was extracted using the Engauge Digitizer software. The methodological quality of all selected studies was evaluated and scored using the Newcastle-Ottawa Scale (NOS), which includes three parts: participant selection, comparability, and outcome (12).
3.5. Statistical Analysis
Stata™ version 12 software was used for data analysis, and Review Manager™ version 5.4 was used for data collection. The dichotomous variables were analyzed using relative risk (RR) with a 95% confidence interval (95% CI), and continuous variables were analyzed utilizing weighted mean difference (WMD) with a 95% CI. P-value < 0.05 was considered statistically significant. Moreover, χ2 and I2 were used to evaluate heterogeneity in these studies (13). According to the relevant standards in the Cochrane Intervention System Evaluation Manual, the fixed-effect method was used if heterogeneity was acceptable (I2 < 50%, P > 0.10). Once heterogeneity was established (I2 ≥ 50% and P ≤ 0.10), the subgroup analyses were further conducted to explore between-study sources of heterogeneity. Descriptive analysis was used in cases where inter-group heterogeneity was excessive or difficult to merge. Publication bias was assessed using Begg’s test and Egger’s test, and P > 0.10 was considered no publication bias.
4. Results
4.1. Selection of Studies
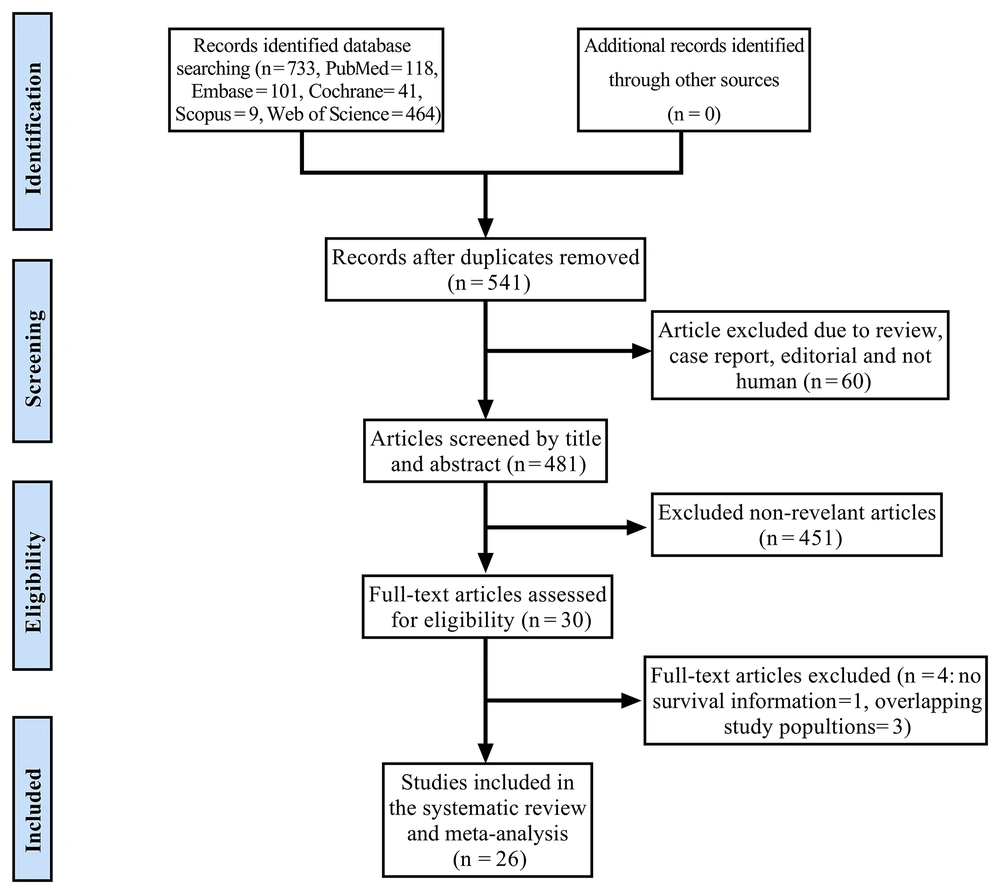
The preliminary systematic search identified 733 relevant studies. After further screening, 30 papers met the inclusion criteria (8, 9, 14-41). Among these studies, four were excluded because of insufficient survival information or overlapping study populations (38-41). Finally, 26 articles involving 20381 participants were identified as eligible papers published during 1997 - 2021. Among these articles, 5, 16, 2, 1and 1 were from China, Japan, the United States, France, Italy, and a multicenter study, respectively. The study identification process is provided in Figure 1 details. The characteristics of the selected studies are presented in Table 1.
| Number | Author | Year | Country | Group | Number of Patients | Gender (Male/ Female) | Age (y) | Median Follow-up (Months) | 5-year OS (%) | 5-year RFS (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Li (8) | 2007 | China | HBV-HCC | 251 | 212/39 | 51.2 ± 4.2 | 51.2 | 64.9 | 62.2 |
| HCV-HCC | 75 | 62/13 | 63.2 ± 7.3 | 38.7 | 35.8 | |||||
| NBNC-HCC | 54 | 44/10 | 67.1 ± 5.7 | 68.5 | 67.7 | |||||
| 2 | Takeishi (9) | 2015 | Japan | HBV-HCC | 94 | 71/23 | 55 ± 11 | -- a | 69.1 | 34.0 |
| HCV-HCC | 451 | 290/161 | 68 ± 8 | -- a | 66.9 | 31.7 | ||||
| NBNC-HCC | 117 | 88/29 | 70 ± 10 | -- a | 64.1 | 34.2 | ||||
| 3 | Utsunomiya (14) | 2015 | Japan | HBV-HCC | 2194 | 1796/398 | 57 | 19.2 | 65.0 | 41.0 |
| HCV-HCC | 7018 | 5225/1793 | 67 | 20.4 | 59.0 | 31.0 | ||||
| NBNC-HCC | 2738 | 2253/485 | 67 | 18.0 | 68.0 | 47.0 | ||||
| 4 | Okuda (15) | 2014 | Japan | HBV-HCC | 32 | 23/9 | 61.1 ± 13.6 | 20 | 49.1 | 29.2 |
| HCV-HCC | 93 | 70/23 | 67.2 ± 6.6 | 65.0 | 27.6 | |||||
| NBNC-HCC | 76 | 67/9 | 71.0 ± 9.2 | 74.1 | 43.8 | |||||
| 5 | Cescon (16) | 2009 | Italy | HBV-HCC | 25 | 24/1 | 60.2 ± 9.8 | 30 | -- a | 9.0 |
| HCV-HCC | 130 | 90/40 | 65.2 ± 8.1 | -- a | 38.0 | |||||
| NBNC-HCC | 35 | 30/5 | 64.2 ± 9.1 | -- a | 34.0 | |||||
| 6 | Chen (17) | 1998 | Taiwan, China | HBV-HCC | 57 | 40/17 | 51.6 ± 12.3 | 12.2 | 40.3 | 24.6 |
| HCV-HCC | 34 | 22/12 | 61.9 ± 10.0 | 38.2 | 29.4 | |||||
| 7 | Chirica (18) | 2013 | France | HBV-HCC | 15 | -- a | -- a | -- a | 46.7 | -- a |
| HCV-HCC | 30 | -- a | -- a | -- a | 38.0 | -- a | ||||
| 8 | Iida (19) | 2015 | Japan | HBV-HCC | 144 | -- a | 60.3 ± 10.8 | -- a | 53.0 | -- a |
| HCV-HCC | 550 | -- a | 69.4 ± 7.6 | -- a | 47.4 | -- a | ||||
| NBNC-HCC | 164 | -- a | 70.3 ± 7.8 | -- a | 54.0 | -- a | ||||
| 9 | Kaibori (20) | 2012 | Japan | HBV-HCC | 85 | 68/17 | 59.3 ± 11.4 | -- a | 53.0 | 32.0 |
| HCV-HCC | 351 | 272/79 | 66.5 ± 7.1 | -- a | 57.0 | 17.0 | ||||
| NBNC-HCC | 60 | 52/8 | 66.6 ± 13.3 | -- a | 62.0 | 35.0 | ||||
| 10 | Kao (21) | 2011 | Taiwan, China | HBV-HCC | 609 | 516/93 | 56.3 ± 13.5 | 40.6 | 52.5 | 36.1 |
| HCV-HCC | 206 | 147/59 | 67.2 ± 9.1 | 55.0 | 35.1 | |||||
| 11 | Lee (22) | 2014 | United States | HBV-HCC | 181 | 147/34 | 56.1 | -- a | 65.0 | 42.0 |
| HCV-HCC | 74 | 58/16 | 62.9 | -- a | 58.0 | 48.6 | ||||
| 12 | Nagasue (23) | 1998 | Japan | HBV-HCC | 15 | 12/3 | 50.6 ± 33.5 | -- a | 84.0 | 84.6 |
| HCV-HCC | 29 | 26/3 | 34.2 ± 26.3 | -- a | 26.0 | 10.7 | ||||
| 13 | Nanashima (24) | 2007 | Japan | HBV-HCC | 76 | 61/15 | 59 ± 11 | -- a | 51.8 | 14.3 |
| HCV-HCC | 124 | 99/25 | 67 ± 7 | -- a | 54.8 | 20.8 | ||||
| NBNC-HCC | 29 | 21/8 | 65 ± 8 | -- a | 53.9 | 44.0 | ||||
| 14 | Nishikawa (25) | 2013 | Japan | HBV-HCC | 62 | 42/20 | 57.7 ± 12.3 | 49.2 ± 36.0 | 63.0 | 22.0 |
| HCV-HCC | 284 | 194/90 | 69.1 ± 7.8 | 49.2 ± 34.8 | 53.0 | 20.0 | ||||
| NBNC-HCC | 129 | 104/25 | 68.6 ± 8.8 | 45.6 ± 34.8 | 63.0 | 26.0 | ||||
| 15 | Pawlik (26) | 2004 | Multi center | HBV-HCC | 163 | 137/26 | 60 | 33 | 38.0 | -- a |
| HCV-HCC | 79 | 49/30 | 60 | 45.6 | -- a | |||||
| NBNC-HCC | 126 | 90/36 | 51 | 33.3 | -- a | |||||
| 16 | Roayaie (27) | 2000 | United States | HBV-HCC | 21 | 10/11 | 54.3 ± 15.3 | -- a | 52.4 | 47.6 |
| HCV-HCC | 24 | 17/7 | 63.4 ± 8.5 | -- a | 33.3 | 8.3 | ||||
| 17 | Sasaki (28) | 2006 | Japan | HBV-HCC | 66 | 49/17 | -- a | -- a | 69.0 | 54.0 |
| HCV-HCC | 351 | 268/83 | -- a | -- a | 62.0 | 24.0 | ||||
| 18 | Tanaka (29) | 2006 | Japan | HBV-HCC | 60 | 51/9 | 56 | 33 | 44.7 | 39.7 |
| HCV-HCC | 137 | 101/36 | 68 | 50.2 | 27.7 | |||||
| NBNC-HCC | 39 | 30/9 | 68 | 61.0 | 67.5 | |||||
| 19 | Wakiyama (30) | 2017 | Japan | HBV-HCC | 36 | 33/3 | 52 ± 9 | -- a | 54.8 | 26.8 |
| HCV-HCC | 57 | 46/11 | 67 ± 7 | -- a | 64.5 | 23.4 | ||||
| NBNC-HCC | 41 | 36/5 | 63 ± 12 | -- a | 71.4 | 44.9 | ||||
| 20 | Wu (31) | 1999 | Taiwan, China | HBV-HCC | 131 | 110/21 | 54.3 | 34.5 | 57.0 | 29.0 |
| HCV-HCC | 70 | 56/14 | 64.1 | 65.0 | 22.0 | |||||
| NBNC-HCC | 40 | 29/11 | 68.9 | 83.0 | 66.0 | |||||
| 21 | Yamanaka (32) | 1997 | Japan | HBV-HCC | 27 | 24/3 | 51 ± 10 | -- a | 52.0 | 60.0 |
| HCV-HCC | 151 | 125/26 | 63 ± 6.3 | -- a | 42.0 | 13.0 | ||||
| NBNC-HCC | 20 | 18/2 | 63 ± 6.4 | -- a | 64.0 | 46.0 | ||||
| 22 | Yamashita (33) | 2014 | Japan | HBV-HCC | 110 | 83/27 | 55 ± 11 | 62.4 | 65.0 | 36.0 |
| HCV-HCC | 474 | 304/170 | 68 ± 8 | 68.0 | 32.0 | |||||
| NBNC-HCC | 110 | 81/29 | 66 ± 10 | 67.0 | 37.0 | |||||
| 23 | Yokoi (34) | 2005 | Japan | HBV-HCC | 25 | 19/6 | 57 | -- a | 59.1 | 40.0 |
| HCV-HCC | 116 | 95/21 | 64 | -- a | 62.1 | 21.6 | ||||
| NBNC-HCC | 13 | 10/3 | 58 | -- a | 100 | 69.2 | ||||
| 24 | Zhang (35) | 2015 | China | HBV-HCC | 409 | 373/36 | -- a | -- a | 35.0 | 34.5 |
| NBNC-HCC | 64 | 53/11 | -- a | -- a | 62.0 | 51.2 | ||||
| 25 | Takenaka (36) | 1995 | Japan | HBV-HCC | 30 | 22/8 | 57 ± 9.4 | -- a | 61.8 | 46.2 |
| HCV-HCC | 96 | 77/19 | 61.7 ± 6.9 | -- a | 52.8 | 23.2 | ||||
| 26 | Ohkura (37) | 2015 | Japan | HBV-HCC | 167 | 144/23 | 54 | -- a | 81.6 | 48.6 |
| HCV-HCC | 401 | 287/144 | 64 | -- a | 74.7 | 34.0 | ||||
| NBNC-HCC | 62 | 46/16 | 68 | -- a | 75.0 | 50.4 |
Abbreviations: HBV-HCC, hepatitis B virus-related hepatocellular carcinoma; HCV-HCC, hepatitis C virus-related hepatocellular carcinoma; NBNC-HCC, non-hepatitis B virus non-hepatitis C virus hepatocellular carcinoma; OS, overall survival; RFS, recurrence-free survival
a Data could not be extracted.
4.2. Patients Characteristics
4.2.1. General Materials
The results from the overall meta-analysis are outlined in Table 2. The mean age of patients with HBV-HCC was significantly lower than that of patients with HCV-HCC and NBNC-HCC (P < 0.05). The incidence rate of male patients in the HCV-HCC group was lower than in the other two groups (P < 0.05).
| Characteristic | Group | Number of Studies | Number of Patients | Results | RR/WMD | 95% CI | P-Value | I2 (%) |
|---|---|---|---|---|---|---|---|---|
| Age (y) | HCV-HCC vs. HBV-HCC | 14 (8, 9, 15-17, 19-21, 24, 25, 30, 32, 33, 36) | 4714 | HBV-HCC = 55.8 ± 11.7 HCV-HCC = 67.3 ± 7.9 | 10.06 | 8.62, 11.51 | 0.000 | 74.8 |
| HBV-HCC vs. NBNC-HCC | 11 (8, 9, 15, 16, 19, 20, 24, 25, 30, 32, 33) | 1777 | HBV-HCC = 55.8 ± 10.4 NBNC-HCC = 68.2 ± 9.3 | -10.52 | -12.83, -8.22 | 0.000 | 82.3 | |
| HCV-HCC vs. NBNC-HCC | 11 (8, 9, 15, 16, 19, 20, 24, 25, 30, 32, 33) | 3575 | HCV-HCC = 67.6 ± 7.8 NBNC-HCC = 68.0 ± 9.6 | -0.34 | -1.65, 0.97 | 0.612 | 70.1 | |
| Male gender | HCV-HCC vs. HBV-HCC | 22 (8, 9, 14-17, 20-28, 30-34, 36, 37) | 15839 | HBV-HCC = 81.6% HCV-HCC = 73.4% | 0.92 | 0.88, 0.95 | 0.000 | 51.4 |
| HBV-HCC vs. NBNC-HCC | 16 (8, 9, 14-16, 20, 24-26, 30-35, 37) | 7909 | HBV-HCC = 82.6% NBNC-HCC = 81.8% | 1.02 | 0.98, 1.07 | 0.297 | 38.6 | |
| HCV-HCC vs. NBNC-HCC | 15 (8, 9, 14-16, 20, 24-, 30-34, 37) | 14238 | HCV-HCC = 73.5% NBNC-HCC = 81.8% | 0.90 | 0.89, 0.92 | 0.000 | 0 | |
| Serum ALT level (IU/L) | HCV-HCC vs. HBV-HCC | 13 (8, 9, 16, 17, 19, 20, 23, 25, 27, 30, 32, 33, 36) | 3663 | HBV-HCC = 48.6 ± 41.9 HCV-HCC = 60.3 ± 43.6 | 16.97 | 12.24, 21.70 | 0.000 | 45.1 |
| HBV-HCC vs. NBNC-HCC | 9 (8, 9, 16, 19, 20, 25, 30, 32, 33) | 1564 | HBV-HCC = 48.1 ± 40.4 NBNC-HCC = 42.5 ± 34.2 | 4.13 | 0.74, 7.52 | 0.017 | 0 | |
| HCV-HCC vs. NBNC-HCC | 9 (8, 9, 16, 19, 20, 25, 30, 32, 33) | 3253 | HCV-HCC = 58.9 ± 42.4 NBNC-HCC = 42.6 ± 34.0 | 21.02 | 13.19, 28.85 | 0.000 | 85.4 | |
| Serum AST level (IU/L) | HCV-HCC vs. HBV-HCC | 10 (9, 16, 17, 19, 23, 25, 30, 32, 33, 36) | 2856 | HBV-HCC = 48.7 ± 44.5 HCV-HCC = 60.9 ± 39.8 | 14.19 | 11.22, 17.17 | 0.000 | 29.5 |
| HBV-HCC vs. NBNC-HCC | 7 (9, 16, 19, 25, 30, 32, 33) | 1114 | HBV-HCC = 46.7 ± 43.0 NBNC-HCC = 42.6 ± 37.5 | 0.51 | -4.75, 5.78 | 0.848 | 43.2 | |
| HCV-HCC vs. NBNC-HCC | 7 (9, 16, 19, 25, 30, 32, 33) | 2713 | HCV-HCC = 59.5 ± 38.8 NBNC-HCC = 46.6 ± 37.5 | 16.63 | 7.71, 25.55 | 0.000 | 87.1 | |
| Serum ALB level (g/dL) | HCV-HCC vs. HBV-HCC | 12 (8, 9, 16, 19, 20, 23, 25, 27, 30, 32, 33, 36) | 3572 | HBV-HCC = 4.0 ± 0.5 HCV-HCC = 3.7 ± 0.4 | -0.25 | -0.34, -0.15 | 0.000 | 84.8 |
| HBV-HCC vs. NBNC-HCC | 9 (8, 9, 16, 19, 20, 25, 30, 32, 33) | 1564 | HBV-HCC = 4.0 ± 0.5 NBNC-HCC = 4.0 ± 0.5 | -0.04 | -0.09, 0.01 | 0.156 | 13.1 | |
| HCV-HCC vs. NBNC-HCC | 9 (8, 9, 16, 19, 20, 25, 30, 32, 33) | 3253 | HCV-HCC = 3.7 ± 0.4 NBNC-HCC = 4.0 ± 0.5 | -0.25 | -0.36, -0.13 | 0.000 | 87.9 | |
| ICG R15 (%) | HCV-HCC vs. HBV-HCC | 8 (9, 19, 20, 23, 30, 32, 33, 36) | 2700 | HBV-HCC = 15.0 ± 10.6 HCV-HCC = 19.9 ± 11.5 | 5.25 | 4.29, 6.21 | 0.000 | 32.0 |
| HBV-HCC vs. NBNC-HCC | 6 (9, 19, 20, 30, 32, 33) | 1008 | HBV-HCC = 15.2 ± 10.8 NBNC-HCC = 15.4 ± 10.2 | -0.26 | -1.49, 0.97 | 0.680 | 0 | |
| HCV-HCC vs. NBNC-HCC | 6 (9, 19, 20, 30, 32, 33) | 2546 | HCV-HCC = 19.9 ± 11.5 NBNC-HCC = 15.4 ± 10.2 | 4.56 | 3.03, 6.10 | 0.000 | 52.7 | |
| Serum T-Bil level (mg/dL) | HCV-HCC vs. HBV-HCC | 12 (8, 9, 16, 17, 19, 20, 23, 25, 27, 30, 33, 36) | 3485 | HBV-HCC = 0.9 ± 0.6 HCV-HCC = 0.9 ± 0.6 | 0.03 | -0.05, 0.12 | 0.413 | 76.3 |
| HBV-HCC vs. NBNC-HCC | 8 (8, 9, 16, 19, 20, 25, 30, 33) | 1517 | HBV-HCC = 0.9 ± 0.4 NBNC-HCC = 0.8 ± 0.4 | 0.11 | 0.004, 0.22 | 0.042 | 83.9 | |
| HCV-HCC vs. NBNC-HCC | 8 (8, 9, 16, 19, 20, 25, 30, 33) | 3082 | HCV-HCC = 0.9 ± 0.6 NBNC-HCC = 0.8 ± 0.4 | 0.11 | 0.01, 0.21 | 0.035 | 87.5 | |
| Platelet count (×103 mm) | HCV-HCC vs. HBV-HCC | 12 (8, 9, 16, 17, 19, 20, 23, 25, 27, 30, 33, 36) | 3485 | HBV-HCC = 174.9 ± 114.2 HCV-HCC = 140.6 ± 200.1 | -27.57 | -41.27, -13.87 | 0.000 | 72.0 |
| HBV-HCC vs. NBNC-HCC | 8 (8, 9, 16, 19, 20, 25, 30, 33) | 1517 | HBV-HCC = 173.5 ± 116.0 NBNC-HCC = 181.5 ± 122.4 | -19.79 | -35.07, -4.51 | 0.011 | 63.7 | |
| HCV-HCC vs. NBNC-HCC | 8 (8, 9, 16, 19, 20, 25, 30, 33) | 3082 | HCV-HCC = 141.2 ± 206.9 NBNC-HCC = 181.5 ± 122.4 | -40.91 | -48.65, -33.17 | 0.000 | 15.1 | |
| Child’s grade A | HCV-HCC vs. HBV-HCC | 13 (8, 14, 17, 19-22, 24, 26, 28, 33, 34, 37) | 13981 | HBV-HCC = 87.7% HCV-HCC = 84.3% | 0.96 | 0.93, 0.99 | 0.007 | 62.9 |
| HBV-HCC vs. NBNC-HCC | 9 (8, 14, 20, 24, 26, 33-35, 37) | 6736 | HBV-HCC = 87.8% NBNC-HCC = 89.9% | 0.97 | 0.91, 1.03 | 0.307 | 85.9 | |
| HCV-HCC vs. NBNC-HCC | 8 (8, 14, 20, 24, 26, 33, 34, 37) | 11830 | HCV-HCC = 85.2% NBNC-HCC = 89.8% | 0.94 | 0.93, 0.96 | 0.000 | 26.7 | |
| Tumor size (cm) | HCV-HCC vs. HBV-HCC | 14 (8, 9, 16, 17, 19, 20, 22, 23, 25, 27, 30, 32, 33, 36) | 3918 | HBV-HCC = 4.4 ± 3.4 HCV-HCC = 3.4 ± 2.2 | -0.78 | -1.12, -0.44 | 0.000 | 72.0 |
| HBV-HCC vs. NBNC-HCC | 9 (8, 9, 16, 19, 20, 25, 30, 32, 33) | 1564 | HBV-HCC = 3.8 ± 2.9 NBNC-HCC = 4.8 ± 3.6 | -0.59 | -1.01, -0.17 | 0.006 | 65.4 | |
| HCV-HCC vs. NBNC-HCC | 9 (8, 9, 16, 19, 20, 25, 30, 32, 33) | 3253 | HCV-HCC = 3.3 ± 2.1 NBNC-HCC = 4.8 ± 3.6 | -1.37 | -1.91, -0.83 | 0.000 | 87.0 | |
| Serum AFP level | HCV-HCC vs. HBV-HCC | 10 (9, 16, 19, 20, 24, 25, 27, 30, 33, 36) | 3224 | HBV-HCC = 8932.6 ± 6456.4 HCV-HCC = 1145.6 ± 7081.8 | -2103.05 | -4445.86, 239.75 | 0.079 | 92.7 |
| HBV-HCC vs. NBNC-HCC | 8 (9, 16, 19, 20, 24, 25, 30, 33) | 1317 | HBV-HCC = 9122.0 ± 66516.2 NBNC-HCC = 4349.1 ± 36879.9 | 2051.21 | -5.26, 4107.68 | 0.051 | 88.7 | |
| HCV-HCC vs. NBNC-HCC | 8 (9, 16, 19, 20, 24, 25, 30, 33) | 3106 | HCV-HCC = 1088.5 ± 6849.7 NBNC-HCC = 4584.5 ± 39144.8 | -650.49 | -1.50, 221.25 | 0.144 | 49.9 | |
| Capsule formation | HCV-HCC vs. HBV-HCC | 10 (8, 15-17, 23, 25, 31, 32, 34, 36) | 2139 | HBV-HCC = 52.8% HCV-HCC = 64.5% | 1.03 | 0.92, 1.16 | 0.587 | 51.9 |
| HBV-HCC vs. NBNC-HCC | 8 (8, 15, 16, 25, 31, 32, 34, 35) | 1566 | HBV-HCC = 61.1% NBNC-HCC = 61.4% | 1.04 | 0.89, 1.21 | 0.616 | 61.8 | |
| HCV-HCC vs. NBNC-HCC | 7 (8, 15, 16, 25, 31, 32, 34) | 1699 | HCV-HCC = 64.1% NBNC-HCC = 58.6% | 1.10 | 0.99, 1.22 | 0.074 | 18.4 | |
| Intrahepatic metastases/ satellite nodules | HCV-HCC vs. HBV-HCC | 9 (9, 14-16, 24, 28, 31, 36) | 1112 | HBV-HCC = 22.3% HCV-HCC = 16.7% | 0.72 | 0.56, 0.91 | 0.007 | 60.9 |
| HBV-HCC vs. NBNC-HCC | 7 (9, 14-16, 24, 31, 34) | 5625 | HBV-HCC = 21.8% NBNC-HCC = 27.3% | 1.18 | 0.73, 1.90 | 0.507 | 75.6 | |
| HCV-HCC vs. NBNC-HCC | 7 (9, 14-16, 24, 31, 34) | 11050 | HCV-HCC = 16.3% NBNC-HCC = 27.3% | 0.71 | 0.54, 0.95 | 0.022 | 43.6 | |
| Vascular invasion | HCV-HCC vs. HBV-HCC | 10 (8, 17, 21-24, 26-28, 32) | 2613 | HBV-HCC = 22.9% HCV-HCC = 21.2% | 0.79 | 0.53, 1.17 | 0.242 | 78.6 |
| HBV-HCC vs. NBNC-HCC | 4 (8, 24, 26, 32) | 746 | HBV-HCC = 15.7% NBNC-HCC = 9.2% | 1.62 | 0.93, 2.83 | 0.090 | 17.4 | |
| HCV-HCC vs. NBNC-HCC | 4 (8, 24, 26, 32) | 658 | HCV-HCC = 12.1% NBNC-HCC = 9.2% | 1.12 | 0.43, 2.89 | 0.813 | 60.0 | |
| Coexisting cirrhosis | HCV-HCC vs. HBV-HCC | 19 (8, 9, 14, 15, 17, 20-22, 24-26, 28, 29, 31-34, 36, 37) | 14984 | HBV-HCC = 48.6% HCV-HCC = 50.0% | 1.09 | 1.01, 1.08 | 0.029 | 63.6 |
| HBV-HCC vs. NBNC-HCC | 14 (8, 9, 14, 15, 20, 24-26, 29, 31-34, 37) | 7071 | HBV-HCC = 49.6% NBNC-HCC = 27.9% | 1.57 | 1.34, 1.84 | 0.000 | 67.7 | |
| HCV-HCC vs. NBNC-HCC | 14 (8, 9, 14, 15, 20, 24-26, 29, 31-34, 37) | 13425 | HCV-HCC = 49.4% NBNC-HCC = 27.9% | 1.67 | 1.50, 1.87 | 0.000 | 45.2 |
Abbreviations: RR, risk ratio; WMD, weighted mean difference; CI, confidence interval; HBV-HCC, hepatitis B virus-related hepatocellular carcinoma; HCV-HCC, hepatitis C virus-related hepatocellular carcinoma; NBNC-HCC, non-HBV non-HCV hepatocellular carcinoma; AFP, alpha-fetoprotein; ALB, serum albumin; ICG R15, indocyanine green retention rate at 15 min
4.2.2. Liver Function
The preoperative serum alanine aminotransferase, aspartate aminotransferase levels, and indocyanine green retention rate at 15 min (ICG R15) were higher in the HCV-HCC group than in the HBV-HCC and NBNC-HCC groups (P < 0.05). The NBNC-HCC group had a lower serum total bilirubin level and a higher platelet count than the other two groups (P < 0.05). The preoperative serum albumin (ALB) level and proportion of Child-Pugh grade A in the HBV-HCC and NBNC-HCC groups were higher than in the HCV-HCC group (P < 0.05).
4.2.3. Tumor Characteristics
The mean tumor size in the NBNC-HCC group was the largest among the three groups (P < 0.05). The incidence of intrahepatic metastases/satellite nodules was lower in the HCV-HCC group than in the other two groups (P < 0.05). The proportion of patients with cirrhosis in the HCV-HCC, HBV-HCC, and NBNC-HCC groups gradually increased (P < 0.05).
4.3. Survival
4.3.1. Five-Year Overall Survival
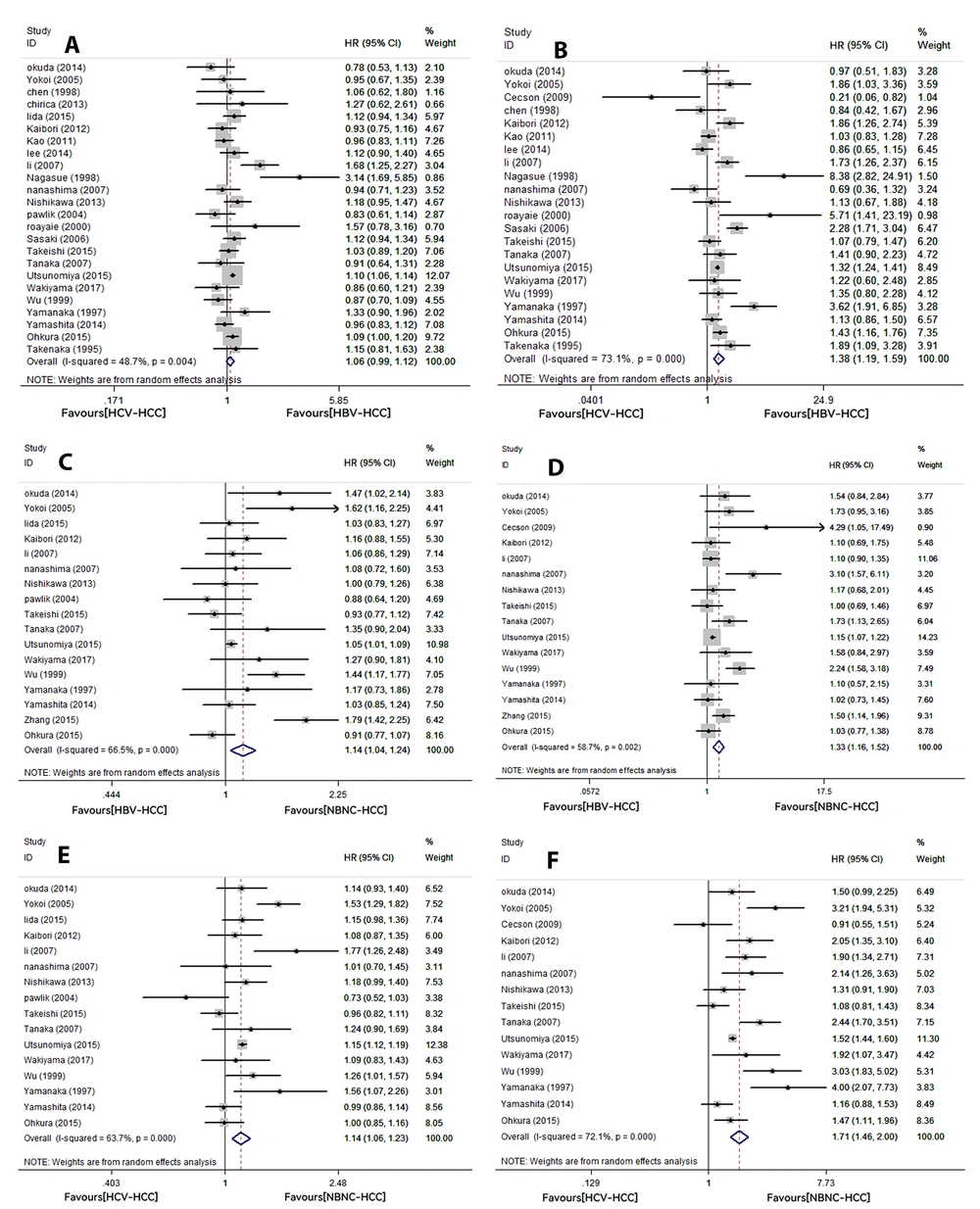
A total of 25 studies reported 5-year OS, including 24 with HCV-HCC and HBV-HCC, 17 with HBV-HCC and NBNC-HCC, and 16 with HCV-HCC and NBNC-HCC. The results showed that 5-year OS was not significantly different between the HCV-HCC and HBV-HCC groups (HR: 1.06, 95% CI: 0.99 - 1.12, P = 0.076). The 5-year OS rates were lower in the HBV-HCC (HR: 1.14, 95% CI: 1.04 - 1.24, P = 0.005) and HCV-HCC groups (HR: 1.14, 95% CI: 1.06 - 1.23, P = 0.001) than in the NBNC-HCC group. The above results showed high heterogeneity (HCV-HCC vs. HBV-HCC, I2: 48.7%, P = 0.004; HBV-HCC vs. NBNC-HCC, I2: 66.5%, P = 0; HCV-HCC vs. NBNC-HCC, I2: 63.7%, P = 0; Figure 2A, C, and E).
4.3.2. Five-Year Recurrence-Free Survival
A total of 23 studies reported 5-year RFS, including 22 with HCV-HCC and HBV-HCC, 16 with HBV-HCC and NBNC-HCC, and 15 with HCV-HCC and NBNC-HCC. There were significant differences in 5-year RFS between patients with different hepatitis virus statuses. Compared to cases with NBNC-HCC, patients with HBV-HCC or HCV-HCC had lower 5-year RFS (HBV-HCC vs. NBNC-HCC, HR: 1.33, 95% CI: 1.16 - 1.52, P = 0; HCV-HCC vs. NBNC-HCC, HR: 1.71, 95% CI: 1.46 - 2, P = 0, respectively). In addition, the 5-year RFS in the HCV-HCC group was lower than in the HBV-HCC group (HR: 1.38, 95% CI: 1.19 - 1.59, P = 0). All the above results showed high heterogeneity (HCV-HCC vs. HBV-HCC, I2: 73.1%, P = 0; HBV-HCC vs. NBNC-HCC, I2: 58.7%, P = 0.002; HCV-HCC vs. NBNC-HCC, I2: 72.1%, P = 0; Figure 2B, D, and F).
4.4. Sensitivity Analysis
The random deletion of the literature in this study had little impact on the results, which means that our results were reasonably robust (Appendix 1).
4.5. Subgroup Analysis According to Hepatitis Virus Status
According to the extracted data and basic information from all selected studies, a subgroup analysis was performed based on the mean tumor size (diameter ≥ 5 cm and diameter < 5 cm), serum alpha-fetoprotein (AFP) level (AFP ≥ 1000 ng/mL and AFP < 1000 ng/mL), serum ALB level (ALB ≥ 4 g/dL and ALB < 4 g/dL), and country. Detailed results are provided in Tables 3 - 5. The observed association between serum AFP levels and the postoperative prognosis was inconsistent among the different subgroups. In the AFP ≥ 1000 ng/mL subgroup, the HCV-HCC group had a lower 5-year OS than the HBV-HCC group (HR: 1.10, 95% CI: 1.06 - 1.14, P = 0). Meanwhile, the 5-year OS rate was lower in the HBV-HCC and HCV-HCC groups than in the NBNC-HCC group (HBV-HCC vs. NBNC-HCC, HR: 1.04, 95% CI: 1.01 - 1.08, P = 0.024; HCV-HCC vs. NBNC-HCC, HR: 1.10, 95% CI: 1.03 - 1.17, P = 0.007). Similarly, virus-related patients had a worse 5-year RFS than patients with NBNC-HCC (HBV-HCC vs. NBNC-HCC, HR: 1.14, 95% CI: 1.07 - 1.21, P = 0; HCV-HCC vs. NBNC-HCC, HR: 1.40, 95% CI: 1.18 - 1.67, P = 0). The mentioned results had low heterogeneity (5-year OS: HCV-HCC vs. HBV-HCC, I2: 0.0%, P = 0.557; HBV-HCC vs. NBNC-HCC, I2: 0.0%, P = 0.747; HCV-HCC vs. NBNC-HCC, I2: 38.0%, P = 0.140; 5-year RFS: HBV-HCC vs. NBNC-HCC, I2:0.0%, P = 0.865; HCV-HCC vs. NBNC-HCC, I2: 58%, P = 0.547). However, the heterogeneity of each group was high in the AFP < 1000 ng/mL subgroup (5-year OS: HCV-HCC vs. HBV-HCC, I2: 67.5%, P = 0.009; HBV-HCC vs. NBNC-HCC, I2: 60.8%, P = 0.037; HCV-HCC vs. NBNC-HCC, I2: 82.0%, P = 0; 5-year RFS: HCV-HCC vs. HBV-HCC, I2: 69.7%, P = 0.006; HBV-HCC vs. NBNC-HCC, I2:55.8%, P = 0.060; HCV-HCC vs. NBNC-HCC, I2: 75.5%, P = 0.003). In addition, the heterogeneity of the mean tumor size, serum ALB level, and country subgroups did not significantly change compared to before grouping. Therefore, these factors were considered to have little correlation with heterogeneity. In summary, AFP had a certain impact on the prognosis of patients with HCC and might be one of the sources of heterogeneity. For those with differences between groups, the results of the two-way comparison were P > 0.05, indicating that they were balanced and comparable. Similarly, no statistically significant differences were observed between subgroups.
| Characteristics and Subgroups | 5-Year OS (HCV-HCC vs. HBV-HCC) | 5-Year RFS (HCV-HCC vs. HBV-HCC) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR [95% CI] | P a | P | Heterogeneity | HR [95% CI] | P a | P | Heterogeneity | |||
| I2 (%) | P | I2 (%) | P | |||||||
| Tumor size (cm) | 0.53 | 0.75 | ||||||||
| ≥ 5 | 0.99 [0.74, 1.33] | 0.969 | 28.8 | 0.246 | 1.50 [0.68, 3.32] | 0.313 | 66.9 | 0.049 | ||
| < 5 | 1.10 [1.00, 1.20] | 0.054 | 65.3 | 0.001 | 1.32 [1.09, 1.58] | 0.003 | 68.6 | 0.000 | ||
| AFP (ng/mL) | 0.33 | 0.77 | ||||||||
| ≥ 1000 | 1.10 [1.06, 1.14] | 0.000 | 0.0 | 0.557 | 1.31 [0.96, 1.75] | 0.069 | 50.8 | 0.071 | ||
| < 1000 | 0.99 [0.81, 1.22] | 0.939 | 67.5 | 0.009 | 1.23 [0.87, 1.72] | 0.239 | 69.7 | 0.006 | ||
| ALB (g/dL) | -- b | -- b | ||||||||
| ≥ 4 | -- b | -- b | -- b | -- b | -- b | -- b | -- b | -- b | ||
| < 4 | 1.04 [0.94, 1.16] | 0.457 | 15.6 | 0.314 | 1.39 [0.86, 2.26] | 0.182 | 79.5 | 0.000 | ||
| Country | 0.73 | 0.56 | ||||||||
| Japan | 1.06 [1.00, 1.13] | 0.065 | 42.5 | 0.037 | 1.49 [1.26, 1.76] | 0.000 | 70.7 | 0.000 | ||
| China | 1.09 [0.82, 1.44] | 0.555 | 78.3 | 0.003 | 1.23 [0.90, 1.70] | 0.196 | 63.4 | 0.042 | ||
| United States | 1.16 [0.94, 1.43] | 0.175 | 0.0 | 0.363 | 1.97 [0.29, 13.23] | 0.486 | 86.1 | 0.007 | ||
Abbreviations: OS, overall survival; RFS, recurrence-free survival; HR, hazard ratio; CI, confidence interval; AFP, alpha-fetoprotein; ALB, albumin; HCV-HCC, hepatitis C virus-related hepatocellular carcinoma; HBV-HCC, hepatitis B virus-related hepatocellular carcinoma
a Between-group statistically significant
b Data could not be extracted.
| Characteristics and Subgroups | 5-Year OS (HBV-HCC vs. NBNC-HCC) | 5-Year RFS (HBV-HCC vs. NBNC-HCC) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR [95% CI] | P a | P | Heterogeneity | HR [95% CI] | P a | P | Heterogeneity | |||
| I2 (%) | P | I2 (%) | P | |||||||
| Tumor size (cm) | 0.13 | 0.10 | ||||||||
| ≥ 5 | 1.20 [0.98, 1.46] | 0.083 | 50.7 | 0.087 | 1.54 [1.07, 2.22] | 0.019 | 59.9 | 0.058 | ||
| < 5 | 1.01 [0.91, 1.11] | 0.900 | 0.0 | 0.800 | 1.09 [0.89, 1.34] | 0.415 | 25.3 | 0.260 | ||
| AFP (ng/mL) | 0.18 | 0.11 | ||||||||
| ≥ 1000 | 1.04 [1.01, 1.08] | 0.024 | 0.0 | 0.747 | 1.14 [1.07, 1.21] | 0.000 | 0.0 | 0.865 | ||
| < 1000 | 1.22 [0.97, 1.53] | 0.086 | 60.8 | 0.037 | 1.52 [1.08, 2.13] | 0.016 | 55.8 | 0.060 | ||
| ALB (g/dL) | 0.09 | 0.43 | ||||||||
| ≥ 4 | 1.04 [1.01,1.08] | 0.024 | 0.0 | 0.979 | 1.14 [1.07, 1.21] | 0.000 | 0.0 | 0.920 | ||
| < 4 | 1.25 [1.02, 1.53] | 0.031 | 0.0 | 0.583 | 1.33 [0.91, 1.95] | 0.142 | 24.3 | 0.266 | ||
| Country | 0.09 | 0.32 | ||||||||
| Japan | 1.07 [0.99, 1.15] | 0.086 | 32.8 | 0.120 | 1.23 [1.07, 1.41] | 0.003 | 34.0 | 0.120 | ||
| China | 1.39 [1.03, 1.87] | 0.030 | 82.9 | 0.003 | 1.51 [1.02, 2.24] | 0.038 | 84.0 | 0.002 | ||
Abbreviations: OS, overall survival; RFS, recurrence-free survival; HR, hazard ratio; CI, confidence interval; AFP, alpha-fetoprotein; ALB, albumin; HBV-HCC, hepatitis B virus-related hepatocellular carcinoma; NBNC-HCC, non -hepatitis B virus non-hepatitis C virus hepatocellular carcinoma
a Between-group statistically significant
| Characteristics and Subgroups | 5-Year OS (HCV-HCC vs. NBNC-HCC) | 5-Year RFS (HCV-HCC vs. NBNC-HCC) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR [95% CI] | P a | P | Heterogeneity | HR [95% CI] | P a | P | Heterogeneity | |||
| I2 (%) | P | I2 (%) | P | |||||||
| Tumor size (cm) | -- b | -- b | ||||||||
| ≥ 5 | -- b | -- b | -- b | -- b | -- b | -- b | -- b | -- b | ||
| < 5 | 1.12 [0.96, 1.30] | 0.139 | 69.4 | 0.011 | 1.28 [1.01, 1.62] | 0.044 | 55.8 | 0.060 | ||
| AFP (ng/mL) | 0.39 | 0.70 | ||||||||
| ≥ 1000 | 1.10 [1.03,1.17] | 0.007 | 38.0 | 0.140 | 1.40 [1.18, 1.67] | 0.000 | 58.0 | 0.547 | ||
| < 1000 | 1.24 [0.94, 1.63] | 0.121 | 82.0 | 0.000 | 1.84 [1.26, 2.69] | 0.002 | 75.5 | 0.003 | ||
| ALB (g/dL) | -- b | -- b | ||||||||
| ≥ 4 | -- b | -- b | -- b | -- b | -- b | -- b | -- b | -- b | ||
| < 4 | 1.25 [1.05, 1.50] | 0.014 | 66.6 | 0.018 | 1.98 [1.33, 2.96] | 0.001 | 74.0 | 0.002 | ||
| Country | 0.17 | 0.20 | ||||||||
| Japan | 1.13 [1.05, 1.21] | 0.001 | 56.6 | 0.006 | 1.69 [1.43, 2.00] | 0.000 | 71.0 | 0.000 | ||
| China | 1.46 [1.02, 2.08] | 0.037 | 68.3 | 0.076 | 2.32 [1.47, 3.65] | 0.000 | 55.4 | 0.134 | ||
Abbreviations: OS: overall survival, RFS: recurrence-free survival, HR: hazard ratio, CI: confidence interval, AFP: alpha-fetoprotein, ALB: albumin
a Between-group statistically significant
b Data could not be extracted.
4.6. Publication Bias
According to the results of Begg’s test and Egger’s test, the 5-year OS of the HBV-HCC vs. NBNC-HCC group, 5-year RFS of the HBV-HCC vs. NBNC-HCC group, and 5-year RFS of the HCV-HCC vs. NBNC-HCC group were accompanied by significant publication bias, whereas little publication bias was detected for the other groups (Table 6). To remove the influence of publication bias on the combined results, the trim-and-fill analysis was used to handle this bias. Finally, the trim-and-fill method adjusted results were consistent with the original analysis, showing that the combined effect was trustworthy (Appendix 2).
| Survival | Group | Begg’s Test | Egger’s Test |
|---|---|---|---|
| Five-year overall survival | HCV-HCC vs. HBV-HCC | 0.309 | 0.699 |
| HBV-HCC vs. NBNC-HCC | 0.044 | 0.132 | |
| HCV-HCC vs. NBNC-HCC | 0.499 | 0.934 | |
| Five-year recurrence-free survival | HCV-HCC vs. HBV-HCC | 0.735 | 0.614 |
| HBV-HCC vs. NBNC-HCC | 0.034 | 0.035 | |
| HCV-HCC vs. NBNC-HCC | 0.048 | 0.201 |
Abbreviations: HBV-HCC: hepatitis B virus-related hepatocellular carcinoma, HCV-HCC: hepatitis C virus-related hepatocellular carcinoma, NBNC-HCC: non-hepatitis B virus non-hepatitis C virus hepatocellular carcinoma.
5. Discussion
Hepatitis B virus and HCV are both hepatotropic viral infections with significantly different molecular carcinogenic mechanisms. The carcinogenic potential of HBV is due to its ability to integrate into the DNA of host cells, directly activate adjacent cellular genes, and provide selective growth advantages for hepatocytes (42). In contrast, HCV is a positive-strand RNA virus that cannot integrate into the genome of liver cells. Therefore, it likely promotes hepatocarcinogenesis through chronic inflammation, liver regeneration, and fibrosis rather than direct carcinogenesis (43). In addition, the carcinogenic mechanism of NBNC-HCC is significantly different from that of virus-related HCC. The exact molecular mechanism for the increase in the NBNC-HCC incidence rate is still indistinct, possibly caused partly by the changing prevalence of non-alcoholic steatohepatitis and metabolic syndrome (1). The difference in the etiology and pathogenesis of HCC might lead to unique clinicopathological characteristics and prognosis in patients with HCC. The present study examined the differences in the postoperative prognosis of HCC with distinct viral statuses and found that the prognosis of patients with NBNC-HCC was superior to patients with HBV-HCC and HCV-HCC in terms of OS and RFS. Meanwhile, patients with HBV-HCC had a more prolonged RFS than patients with HCV-HCC. Furthermore, our research found that HBV-HCC, HCV-HCC, and NBNC-HCC differed significantly in terms of clinical features, liver function, and tumor characteristics.
Previous studies compared the prognostic differences between virus-related HCC and NBNC-HCC with inconsistent results (10, 14, 44). A retrospective single-center study reported that viral hepatitis did not significantly affect long-term prognosis after surgical treatment in patients with HCC (44). A meta-analysis of 4744 cases showed that patients with NBNC-HCC had better 5-year DFS and a tendency toward higher 5-year OS (not statistically significant) than HCC patients with HBV or HCV infection. However, there was no difference in survival between the HBV-HCC and HCV-HCC groups (10). Another large-sample study on 11950 cases revealed that the NBNC-HCC group had a significantly lower risk of recurrence than the HBV-HCC and HCV-HCC groups (14). Similarly, our data from 20381 patients confirmed that patients with NBNC-HCC had better 5-year OS and RFS than those with HBV-HCC or HCV-HCC. Cancer-promoting factors in the inflammatory microenvironment of hepatitis result in gene mutation and chromosomal instability in hepatocytes, leading to intrahepatic recurrence, which is the main cause of death in patients with HCC postoperatively (45, 46). Therefore, we believe that the improved survival rate of NBNC-HCC is because of the absence of a chronic viral attack, and patients with NBNC-HCC maintained good liver function following the initial hepatectomy. These biological advantages provide more opportunities for antitumor treatment (34). Furthermore, we found that the 5-year RFS rate of patients with HCV-HCC was lower than patients with HBV-HCC. Franssen et al. (47) found that the postoperative recurrence rate of HBV-HCC was lower than that of HCV-HCC. Previous studies demonstrated that HCC with different viral etiologies has unique molecular signatures and immune landscapes. Compared to HBV-HCC, HCV-HCC leads to the downregulation of T-cell-related genes within the tumor and the upregulation of oxidative stress genes outside the tumor, and the persistent necrotic inflammatory environment might cause recurrence (48). Consequently, effective therapy could prevent postoperative recurrence and improve the OS of patients with virus-related HCC through viral inhibition and anti-inflammatory effects (49). A large prospective study published in 2021 indicated that direct-acting antiviral (DAA) medication enhanced the survival of patients with HCV-HCC after liver transplantation and was comparable to patients with HBV-HCC (50).
Our analysis indicated that the mean age of patients in the HBV-HCC group was the lowest compared to those in the HCV-HCC and NBNC-HCC groups because chronic HBV infections usually result from the maternal vertical transmission, whereas HCV is mainly caused by blood transmission, such as drug abuse and transfusion, mainly in adulthood. Similarly, NBNC-HCC mainly occurs with long-term excessive drinking and metabolic syndrome for decades. The latter finding was consistent with a previous study that the age of onset in patients with HBV-HCC was lower than in patients with HCV-HCC and NBNC-HCC (10). It is well known that the HCC incidence and mortality rates in men are significantly higher than in women worldwide (1). Our data showed that the proportion of male patients was lower in the HCV-HCC group than in the other two groups. Previous investigations have also reported that the proportion of male patients was smaller for the HCV-HCC than for the HBV-HCC or NBNC-HCC groups (51, 52). Gender significantly contributes to the shape of immune responses and initiates differences in the pathogenesis of infectious diseases (53). Androgens directly interact with the HBV genome integrated into the cell nucleus and activate the transcription of HBV oncoproteins (54). These findings may partially explain the male gender preference for HBV-related HCC.
The finding that the HCV-HCC group had the lowest liver reserve function and the highest ratio of cirrhosis among the three groups was similar to the conclusions of previous studies (10, 14). The complex interaction between HCV and host proteins can cause host responses, increase inflammation and fibrosis, and finally lead to liver cirrhosis (55).
Consistent with the results of Watabe et al. (56), the tumor diameter in the NBNC-HCC group was significantly larger than in the hepatitis virus-related HCC group, which may be attributed to the lack of a regular review of liver diseases in patients with NBNC-HCC. Tumors in patients with NBNC-HCC might be detected only when the tumors are enlarged and cause subjective symptoms. The proportions of vascular invasion and tumor capsule formation were not significantly different between the three etiology-related HCC cases, which is also in line with previous meta-analyses (10). In addition, a published meta-analysis showed that the incidence of intrahepatic metastasis and satellite nodules was similar in the three etiology-related HCC, while we found the incidence of intrahepatic metastasis and satellite nodules in the HCV-HCC group was lower than in the HBV-HCC and NBNC-HCC groups. The high incidence of intrahepatic metastasis in the HBV-HCC group is probably related to the biological behavior of HBV. The expression of HBx in HBV-related hepatocytes can activate the Wnt/β-catenin signaling pathway and further promote the development and progression of HCC (57).
We found that heterogeneity among the groups significantly changed when AFP was used as the grouping basis after subgroup analysis for tumor diameter, AFP, ALB, and country, suggesting that AFP had a certain impact on the prognosis of patients. The same result that AFP level affects the prognosis of patients with HCC was also reported by Al-Ameri et al. (58). Many factors, including surgical margin, vascular invasion, capsule integrity, transarterial chemoembolization, antiviral treatment, and targeted therapy, might initiate heterogeneity, making the survival analysis for patients with the HCC of various etiologies challenging to calculate.
This study had some limitations. First, because of the important heterogeneities between the investigations, we adopted the random-effects model to eliminate the effect of heterogeneity partially. Second, all our analyzed data came from retrospective studies with inherent limitations and inevitable selection bias. Third, since HCC studies without the evaluation of survival were excluded, there might be a certain degree of bias in the results of HCC characteristics. However, the large sample size of this review (26 studies on 20381 participants) is reasonable and feasible to represent the clinical characteristics of the HCC patients of diverse etiologies to a large extent. Fourth, most studies included here were Asian (5 from China and 16 from Japan) owing to the highest incidence rates in China and other parts of East Asia. As a result, the data may not be extrapolated to the non-Asian population. Fifth, the influence of confounding factors from three small studies (40 - 50 patients) cannot be excluded. Finally, this review was not registered, but this meta-analysis was carried out strictly with the PRISMA statement. Based on the above limitations, multicenter, high-quality, and large-sample-size studies must be discussed further.
This study indicated that patients with NBNC-HCC had a significantly better postoperative prognosis than patients with virus-related HCC. Although the 5-year RFS rate of HBV-HCC was higher than HCV-HCC, the difference was relatively small. Moreover, the AFP levels correlated with the postoperative prognosis of patients with HCC. In addition to the tumor stage or hepatic reserve function, our findings suggest that treatment screening for HCC should also be based on hepatitis viral infection status. Antiviral therapy may be a cost-effective and efficient strategy to improve the prognosis of patients with virus-related HCC.