1. Background
Hepatitis A virus (HAV), the most common cause of viral hepatitis, is one of the worldwide health issues (1). This virus is transmitted through the fecal-oral route and causes debilitating symptoms and rarely acute fulminant hepatitis, often fatal. However, it does not cause chronic hepatitis (1-4). The World Health Organization (WHO) showed an increase in acute hepatitis A cases from 117 million in 1990 to 126 million in 2005, and hepatitis A-related deaths from 30,283 in 1990 to 35,245 in 2005 (3). The last worldwide estimates showed that hepatitis A caused approximately 11000 deaths in 2015 and 7134 deaths in 2016 (5). Overall, hepatitis A is generally self-limited, but its mortality rises by age from 0.1% in children < 15 years old to 2.1% in adults over 40 years old (3). Although a safe and effective vaccine is available against HAV, the strategy for vaccination should be based on the endemicity status and cost-effectiveness. According to WHO protocol, universal vaccination is only recommended in HAV intermediate-endemicity areas. In low- or very low-endemic areas, vaccination is recommended only in high-risk groups, such as patients with chronic liver diseases or coagulopathy, travelers to highly-endemic areas, intravenous drug abusers, and homosexuals (1, 3).
2. Objectives
As the first subnational HAV-immunity serosurvey, we conducted this study to determine the prevalence of chronic immunity and endemicity of HAV in all age groups (except infants) in Fars province, Iran. Furthermore, we used both WHO and age at mid-point of population immunity (AMPI) (6, 7) recommended protocols for endemicity detection. The results of this study could be helpful for health policymakers in choosing the best strategy regarding HAV vaccination in this region.
3. Methods
3.1. Sample Size and Sampling
With a 5 million population, Fars province is the fourth largest and populous province of Iran that has diversity in climate and ethnicities. This province is almost a representative of the whole country of Iran. Therefore, we selected this province for detecting HAV-IgG seroprevalence. The statistical population of this study consisted of all age-gender groups (except infants). The sample size in this study was calculated as 576 using the Cochrane formula and considering an anti-HAV immunity level of 50% (1), error of 5%, confidence interval of 95%, drop out of 25%, and design effect of 1.2. In the next step, using multi-stage random sampling, we divided all 31 cities of Fars province into three groups, including cities with a population > 200,000, 50000 - 200000, and < 50000. Next, four cities were randomly chosen from each group. We defined the sample size separately based on the population of each city and considered urban and rural areas and the proportion of age groups in both genders. Sampling was performed in two age groups of children < 15 years and individuals ≥ 15 years old in urban areas.
Sampling from the first group was accomplished in the referral orthopedic (Chamran) hospital affiliated with Shiraz University of Medical Sciences, to which traumatic patients are referred from all parts of Fars province. This strategy was adopted due to difficulties in accessing and taking blood samples from children in urban areas. About the 2nd group, from each randomly selected postal code received from Fars central post office, one person was invited by a posted written invitation to come into the related leading clinic affiliated with the medical university. For sampling in rural areas, we randomly selected two villages out of all the villages of each chosen city. The required samples were selected utilizing the systemic randomization method in the healthcare centers of chosen villages. Afterwards, one person ≥ 1-year-old was invited from each selected family to come to the related health center for an interview and to take a blood sample. There was no exclusion criterion except non-willingness to join this study and age < 1 year who were excluded due to the possibility of congenital HAV immunity.
3.2. Data Collection
For data collecting, face-to-face interviews were performed individually with each person or the parents of children < 15 years using a checklist which consisted of items about demographic characteristics, hand hygiene, access to sanitary toilets and safe water, sanitary disposal of waste, proper disinfection of fruits and vegetables, history of traveling abroad. After filling the checklist, a 3 cc blood sample was taken from each participant, centrifuged in the nearest laboratory, and sent to the Gastrointestinal Laboratory affiliated with Shiraz University of Medical Sciences while observing cold-chain carrying standards. Samples were frozen at -80°C until analysis by enzyme-linked immunosorbent assay (ELISA) using commercial kits for IgG detection (Dia. pro, Italy) (8).
3.3. Statistical Analysis
Data were analyzed using the IBM® SPSS® Statistics version 21. Univariable analysis was completed using the chi-squared or t-test for nominal and continuous variables, respectively. Among independent factors, those with a P-value of < 0.3 were selected and entered into the binary logistic regression model. A backward Wald elimination technique was applied for developing the model, while P < 0.05 was considered significant. Moreover, we measured AMPI, the youngest age at which half of the non-vaccinated members of a birth cohort had developed immunity to HAV as a result of infection, according to one study that introduced a new method for imputing country-level estimates of HAV endemicity levels in the Eastern Mediterranean region (6, 7). Therefore, for determining AMPI, we put the age groups on the y-axis and the percentage of immunity on the x-axis, and the first group with anti-HAV 50% was determined. Next, HAV immunity in each age group was also defined.
The geographical variations of hepatitis A chronic immunity in Fars province were also analyzed by the Bayesian spatial model. We accordingly used the Besag, York, and Mollie (BYM) model (9). Furthermore, OpenBUGS program version 3.2.3 (10) was utilized to estimate parameters and ArcGIS version 10.1 (11) to display the results on maps. We ran two chains with 1000 samples as burn-in and 10000 samples as iterations. Convergence of the chains was confirmed by auto-correlations, trace, and density plots.
4. Results and Discussion
This study was conducted to determine the prevalence of chronic immunity and endemicity of HAV in almost all age groups in Fars province, Iran. Overall, 95% (547 of 576 calculated sample size) of individuals participated in this study with a mean age of 33.07 ± 15.1 and an age range of 1 - 82 years. Our results showed that 296 participants were female and 251 were male, with a female to male ratio of 1.1. We observed that 325 (59.4%) people were married, 175 (32%) had a university education, 208 (38%) were employed, 109 (19.9%) had a history of traveling aboard, and 452 (82.6%) had access to sanitary water. Overall, 380 out of 547 participants (69.4%) had anti-HAV IgG and were chronically immune to this virus. The AMPI was calculated as 25 years old.
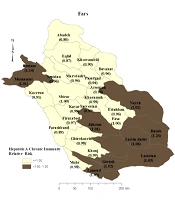
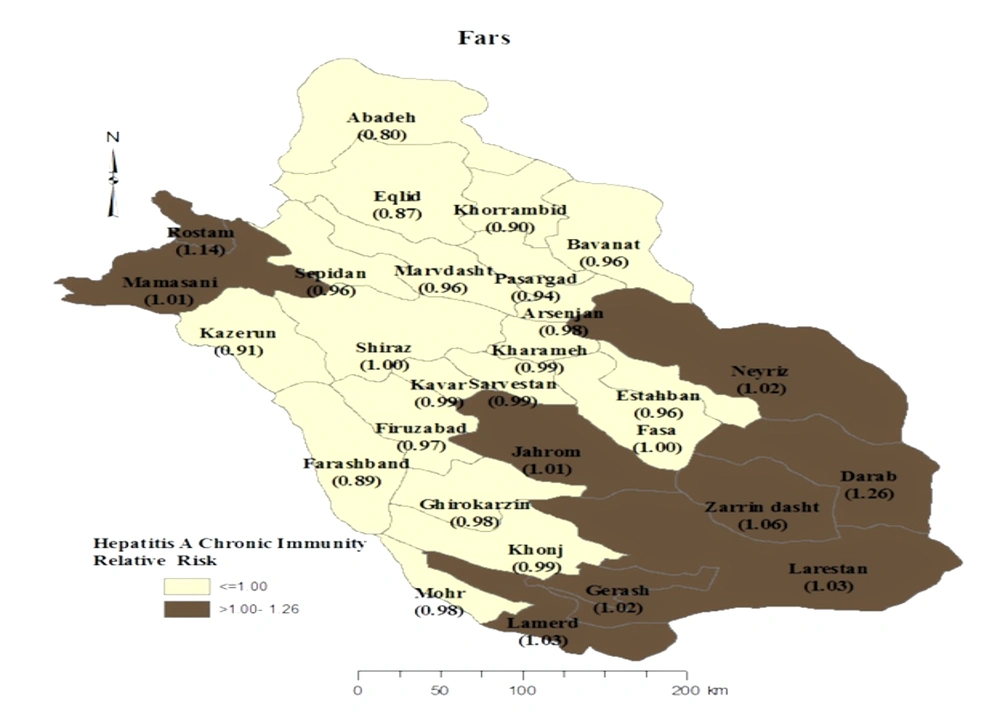
The univariable analysis showed that being older, married, non-Fars ethnicities, employed, knowledgeable about the transmission routes of HAV have a high body mass index (BMI), have a history of travel abroad, the lack of sanitary disposal of sewage or access to sanitary water in living area, and washing or disinfecting fruits and vegetables had a positive association with anti-HAV IgG seropositivity (P < 0.3). Therefore, these variables were entered into multivariate analysis (Table 1). According to the results of logistic regression, being married (OR = 10.7, 95% CI: 6.7 - 17.1), non-Fars ethnicity (OR = 2.8, 95% CI: 1.5 - 5.3), knowledgeable about the transmission routes of HAV (OR = 2.2, 95% CI: 1.2 - 4.2), and employed (OR = 1.7, 95% CI: 1 - 2.7) were the strongest determinants of anti-HAV IgG seropositivity. Figure 1 demonstrates that Darab city in the south of Fars province had the highest prevalence of HAV immunity, while Abadeh had the lowest prevalence. These two areas are among the hottest and coldest cities in this province, respectively.
| Variables | HAV-IgG Negative (n = 167) | HAV-IgG Positive (n = 380) | OR | 95% Cl | P-Value |
|---|---|---|---|---|---|
| Age (y) | 19.85 ± 8.14 | 38.88 ± 13.73 | - | - | < 0.001 |
| Gender | 0.83 | 0.57 - 1.19 | 0.317 | ||
| Male | 82 (32.7) | 169 (67.3) | |||
| Female | 85 (28.7) | 211 (71.3) | |||
| Age Group (y) | - | - | < 0.001 | ||
| < 22 | 106 (77.4) | 31 (22.6) | |||
| 22 - 30 | 49 (34.3) | 94 (65.7) | |||
| 31 - 43 | 11 (8) | 126 (92) | |||
| > 43 | 1 (0.8) | 129 (99.2) | |||
| Education (y) | 0.84 | 5.86 - 14.62 | 0.371 | ||
| ≤ 12 | 111 (30.4) | 254 (69.6) | |||
| ≥ 12 | 56 (32) | 119 (68) | |||
| Marital status | 9.26 | 0.068 - 0.17 | < 0.001 | ||
| Married | 35 (10.8) | 290 (89.2) | |||
| Single | 95 (52.8) | 85 (47.2) | |||
| Ethnicity | 0.45 | 0.26 - 7.83 | 0.005 | ||
| Fars | 117 (28.3) | 297 (71.7) | |||
| Others | 13 (14.3) | 78 (85.7) | |||
| Place of living | 1.03 | 0.71 - 1.48 | 0.871 | ||
| Shiraz (central of Fars) | 83 (30.9) | 186 (69.1) | |||
| others | 84 (30.2) | 194 (69.8) | |||
| BMI (kg/m2) | 162 (22.21 ± 5.02) | 373 (25.83 ± 4.93) | 1.17 | 1.12 - 1.22 | < 0.001 |
| Job Status | 2.01 | 1.35 - 2.99 | < 0.001 | ||
| Not employed | 120 (36.4) | 210 (63.6) | |||
| Employed | 46 (22.1) | 162 (77.9) | |||
| Having supplementary insurance | 0.65 | 0.22 - 1.85 | 0.423 | ||
| Yes | 161 (30.3) | 371 (69.7) | |||
| No | 6 (40) | 9 (60) | |||
| Travel to Muslim countries with holy shrines | 2.7 | 1.57 - 4.66 | < 0.001 | ||
| Yes | 18 (16.5) | 91 (83.5) | |||
| No | 143 (34.9) | 267 (65.1) | |||
| Travel to non-holy shrines countries | 3.48 | 1.69 - 7.19 | 0.001 | ||
| Yes | 9 (12.5) | 63 (87.3) | |||
| No | 158 (33.3) | 317 (66.7) | |||
| House ownership | 1.17 | 0.78 - 1.76 | 0.425 | ||
| Owner | 118 (29.6) | 281 (70.4) | |||
| Tenant | 49 (33.1) | 99 (66.9) | |||
| Household number | 4.02 ± 1.14 | 4.06 ± 1.53 | - | - | 0.781 |
| Having access to sanitary water in the living area | 0.52 | 0.29 - 0.91 | 0.024 | ||
| Yes | 150 (32.5) | 312 (67.5) | |||
| No | 17 (20) | 68 (80) | |||
| Having access to a sanitary toilet in the living area | 2.26 | 0.14 - 36.43 | 0.564 | ||
| Yes | 166 (30.6) | 376 (69.4) | |||
| No | 1 (50) | 1 (50) | |||
| Type of disposing of sewage in the living area | 1.29 | 0.86 - 1.95 | 0.211 | ||
| Municipal sewage (sanitary) | 124 (32.1) | 262 (67.9) | |||
| others | 43 (26.7) | 118 (73.3) | |||
| Sanitary disposing of trash in the living area | 1.32 | 0.26 - 6.64 | 0.73 | ||
| Yes | 162 (30.7) | 366 (69.3) | |||
| No | 2 (25) | 6 (75) | |||
| Hand washing after toilet | 1.17 | 0.61 - 2.24 | 0.616 | ||
| Yes | 153 (30.8) | 343 (69.2) | |||
| No | 14 (27.5) | 37 (72.5) | |||
| Cooking of seafood | - | - | 0.9 | ||
| Yes, completely | 139 (30.4) | 318 (69.6) | |||
| Yes, incompletely | 9 (34.6) | 17 (65.4) | |||
| Not using seafood | 19 (30.2) | 44 (69.8) | |||
| Washing or disinfecting of fruits | 1.21 | 0.84 - 1.75 | 0.291 | ||
| Yes | 89 (28.7) | 221 (71.3) | |||
| No | 78 (32.9) | 159 (67.1) | |||
| Washing or disinfecting of vegetable | 1.54 | 0.97 - 2.42 | 0.062 | ||
| Yes | 129 (28.8) | 319 (71.2) | |||
| No | 38 (38.4) | 61 (61.6) | |||
| The source of edible ice | 0.76 | 0.42 - 1.4 | 0.392 | ||
| sanitary | 151 (31.1) | 334 (68.9) | |||
| not sanitary | 16 (25.8) | 46 (74.2) | |||
| Knowledge about transmission routes of HAV | 1.52 | 0.88 - 2.63 | 0.129 | ||
| Correct | 19 (20.7) | 73 (79.3) | |||
| Incorrect | 122 (28.4) | 307 (71.6) |
Abbreviations: HAV, hepatitis A virus; IgG, immunoglobulin G; OR, odds ratio; CI, confidence interval; BMI, body mass index.
In summary, about two-thirds of people in Fars province are immune to HAV, which along with the lack of an HAV immunization program, shows a herd immunity due to past exposure to this virus. However, immunity against HAV in this province has decreased over the past ten years (12), which indicates the transition of endemicity in this area. The AMPI in Fars province is 25 years old, demonstrating an intermediate endemic status, and the province is categorized as having very low endemicity based on WHO protocol (3, 6). This transition and endemicity status emphasized the potential of HAV outbreaks in Fars province if the surveillance of this virus is neglected (6), especially in the north and center areas which had a lower degree of immunity against this virus. A systematic review in Iran revealed that the overall HAV seroprevalence was 51% - 66% and concluded that Iran is in the low/very low level of HAV endemicity (1). Furthermore, research in Hormozgan province, Iran, which neighbors Fars, showed the high prevalence of HAV in that area which increases the risk of HAV outbreaks in Fars, especially in borderline regions with other provinces to which people frequently commute (13).
5. Conclusion
Fars province should be considered as a very low HAV endemic area according to WHO protocol and as an intermediate HAV endemic area based on AMPI measuring method. However, the strategy of HAV vaccination should be tailored to the results of cost-effectiveness studies and the national HAV vaccination strategy. Furthermore, interventional approaches should prioritize high-risk groups, including educational, sanitary, and immunization programs.