1. Background
Non-alcoholic fatty liver disease (NAFLD) is increasingly the most common liver disease, with a prevalence of 25 - 30% in the general global population (1, 2). The pathologic spectrum of NAFLD ranges from simple steatosis to non-alcoholic steatohepatitis (NASH), which can advance to hepatic fibrosis and ultimately to cirrhosis (3). The NAFLD is the hepatic component of the metabolic syndrome and has been reported to be closely associated with greater adiposity, hyperlipidemia, diabetes, cardiomyopathy, cardiac arrhythmias, and coronary heart disease (CHD) risk (2, 4-8).
Although CHD mortality has decreased in several developed countries, it remains the most serious global public health challenge (9, 10). Recently, several studies investigated coronary artery calcification (CAC) and demonstrated it to be strongly related to CHD incidents predicting adverse clinical outcomes (11-15). The prevalence of CHD was higher in patients with moderate to severe steatosis than in patients with mild steatosis (16). However, the association between fibrosis severity in NAFLD and the degree of CAC is still not clear (17, 18). In this hypothesis-generating study, we examined the association between NAFLD severity and CAC using the Gensini score to prevent or slow the progression of CHD. Therefore, the main aim of the present study was to investigate whether NAFLD extent is linked to the severity of CAC angiographically evaluated using the Gensini score. The NAFLD is commonly diagnosed by abdominal ultrasound. Furthermore, the Fibrosis-4 (FIB4) score, a non-invasive assessment (19), has been used in the current clinical study to predict hepatic fibrosis severity in NAFLD.
2. Objectives
The current study aimed to determine the association between NAFLD severity and coronary stenotic lesions in the eastern Chinese population.
3. Methods
3.1. Subjects
This retrospective cross-sectional study was carried out in the affiliated Hospital of Jiaxing University, Zhejiang, China, in accordance with the Helsinki Declaration. The procedures were evaluated and approved by the Institutional Ethical Board (NO.2017-157). The data were obtained from the records of patients referred to the Department of Cardiovascular Diseases inpatient unit to determine coronary arteriography between January and December 2016. The height and weight of patients were measured, and the body mass index (BMI) was calculated. Lifestyle habits, blood pressures, physical examinations, laboratory assays, and imaging results were recorded. The inclusion criteria were the age range of 18 - 85 years and coronary artery stenosis less than 50% demonstrated by coronary angiography and assessed by at least two expert operators. The exclusion criteria entailed a significant history of alcohol consumption of more than 30 g/day for male or 20 g/day for female participants, any known chronic liver disease, positive results for HBsAg or anti-HCV, hemochromatosis, autoimmune hepatitis, Wilson disease, a history of cardiovascular diseases (eg, heart failure, myocardial infarction, atrial fibrillation, coronary revascularizations, or any other heart disease), renal disease, and malignancies.
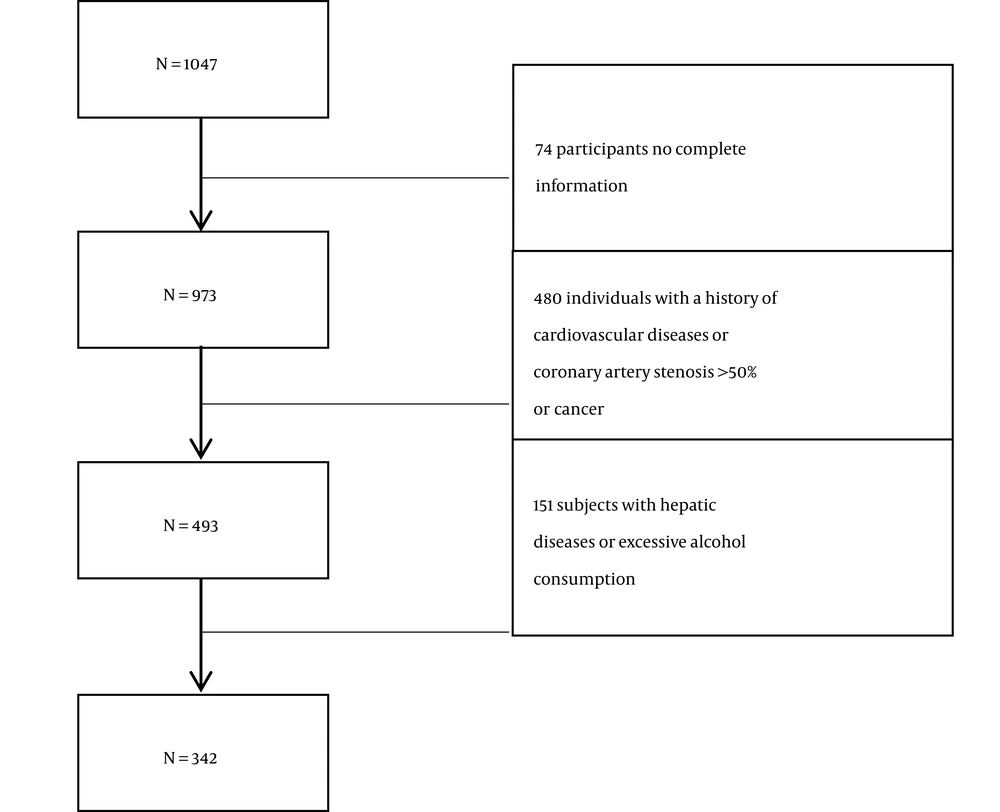
Coronary angiography was initially performed on a total of 1047 participants. The group included 74 participants who did not have complete information, 480 individuals who had a history of cardiovascular diseases, coronary artery stenosis > 50%, or cancer, and 151 participants who had hepatic diseases or excessive alcohol consumption. Finally, 342 patients were eligible for the study after excluding these subjects (Figure 1).
3.2. Laboratory Measurements
Venous blood samples were taken in the morning after an overnight fast by professionally trained nurses. Afterwards, the serum levels of the markers of liver function, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), triglycerides (TG), total cholesterol (TC), Low-density lipoprotein (LDL), high-density lipoprotein (HDL), as well as the marks of kidney function, namely creatinine (Cr) and uric acid (UA), were measured using standard methods by automated analyzers.
3.3. Gensini Score
Gensini score (20) was calculated based on the narrowing degree of the diseased vessels. This gave scores of 1, 2, 4, 8, 16, and 32 points for the reductions of 25%, 50%, 75%, 90%, 99%, and complete occlusion, respectively. A multiplied number was then assigned to different principal vascular segments according to the functional significance of the myocardial area supplied by that segment, and the sum of all points was evaluated. It was found that the higher the score, the narrower the coronary arteries.
3.4. FIB4 Score
The FIB4 score was calculated based on the laboratory data and using the following formula:
In the current study, a FIB4 index less than 1.3 showed a 90% negative predictive value for advanced fibrosis to exclude severe fibrosis. On the contrary, a FIB4 index higher than 2.67 would have a positive predictive value of 80% for advanced fibrosis (21). According to the cut-off value, it was defined as mild, moderate, or severe NAFLD.
3.5. Statistical Analysis
The baseline characteristics of patients studied in the present study were presented as mean ± SD for data with normal distribution and as median and range for continuous variables with non-normal distribution. Furthermore, the normality of data distribution was tested by the Shapiro-Wilk normality test. Comparisons of the parameters between the patients with NAFLD and non-NAFLD were performed using appropriate statistical methods, such as Pearson’s chi-squared test, unpaired t-test, or Mann-Whitney U-test. The correlations between non-invasive FIB4 and Gensini scores were analyzed using the Spearman test. The association of NAFLD severity with coronary artery stenosis or the number of diseased vessels was assessed using the analysis of variance (ANOVA) and LSD post-hoc test. All significance tests were 2-tailed, and P-value < 0.05 was considered statistically significant. All data were analyzed using the IBM SPSS Statistics version 20.0 software (SPSS Inc., IBM, NY, USA).
4. Results
4.1. Characteristics of the Study Population
A total of 1047 participants were selected, and 342 people met the inclusion criteria for the present study (Figure 1). The baseline clinical and main biochemical characteristics of the study population are shown in Table 1. Among 342 enrolled participants, 30.7% (n = 105) were in NAFLD group, and 69.3% (n = 237) were in the control group. Compared to the control group, participants with NAFLD were older and had higher BMI, blood pressure (SBP and DBP), TC, AST, GGT, LDL, Cr, and platelet count. However, the differences were not statistically significant.
| Characteristics | Control (N = 237) | NAFLD Group (N = 105) | P-Value |
|---|---|---|---|
| Age (y) | 59.17 ± 9.113 | 59.84 ± 9.459 | 0.542 |
| Gender (male) | 110 (46.414) | 54 (51.429) | 0.392 |
| BMI (kg/m2) | 22.22 ± 5.446 | 24.72 ± 5.794 | 0.065 |
| SBP (mmHg) | 137.77 ± 21.125 | 138.17 ± 19.595 | 0.864 |
| DBP (mmHg) | 80.61 ± 11.419 | 81.9 ± 12.63 | 0.368 |
| TC (mmol/L) | 4.04 ± 0.932 | 3.92 ± 0.961 | 0.305 |
| TG (mmol/L) | 1.39 ± 0.697 | 1.80 ± 1.002 | <0.001 |
| FBG (mmol/L) | 5.17 ± 1.046 | 5.62 ± 1.068 | <0.001 |
| ALT (IU/L) | 20.22 ± 16.712 | 24.58 ± 17.471 | 0.032 |
| AST (IU/L) | 19.86 ± 13.782 | 20.61 ± 11.892 | 0.612 |
| GGT (IU/L) | 30.15 ± 38.099 | 35.65 ± 29.73 | 0.151 |
| LDL (mmol/L) | 2.48 ± 0.763 | 2.37 ± 0.744 | 0.229 |
| HDL (mmol/L) | 1.19 ± 0.261 | 1.1 ± 0.253 | 0.006 |
| Albumin (g/L) | 41.43 ± 3.902 | 41.54 ± 3.023 | 0.771 |
| Cr (µmol/L) | 70.55 ± 16.159 | 73.65 ± 20.102 | 0.165 |
| UA (µmol/L) | 309.24 ± 82.096 | 339.31 ± 81.06 | 0.002 |
| Platelet count (109/L) | 185.72 ± 56.646 | 186.73 ± 52.241 | 0.873 |
| Diabetes | 21 (8.9) | 26 (24.8) | <0.001 |
| Hypertension | 123 (51.9) | 75 (71.4) | 0.001 |
| Gensini score | 1.69 ± 2.427 | 2.63 ± 3.508 | 0.033 |
Abbreviations, BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; TG, triglycerides; FBG, fasting blood glucose; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; LDL, low-density lipoprotein; HDL, high-density lipoprotein; Cr, creatinine; UA, Uric acid; NAFLD, non-alcoholic fatty liver disease.
a Values are presented as mean ± SD or No. (%).
The serum levels of TG, fasting blood glucose (FBU), ALT, and UA were significantly higher in patients with NAFLD than in participants without NAFLD (1.80 ± 1.002 vs. 1.39 ± 0.697, 5.62 ± 1.068 vs. 5.17 ± 1.046, 24.58 ± 17.471 vs. 20.22 ± 16.712, and 339.31 ± 81.06 vs. 309.24 ± 82.096; P < 0.001, P < 0.001, P = 0.032, and P = 0.002). Moreover, patients with NAFLD have a higher percentage of hypertension and diabetes mellitus (P < 0.001 and P = 0.001) than participants without NAFLD. On the other hand, HDL level was lower in patients with NAFLD than participants without NAFLD (1.1 ± 0.253 vs. 1.19 ± 0.261, P = 0.006). In addition, Gensini scores were higher in patients with NAFLD than in participants without NAFLD (1.69 ± 2.427 vs. 2.63 ± 3.508, P = 0.033), as shown in Table 1.
4.2. Association between NAFLD Rate and the Number of Coronary Lesions
Patients with CAC were further divided into four groups (control, single-vessel, two-vessel, and multi-vessel) depending on the number of diseased arteries. The NAFLD rate was also compared between these different groups in the current study. It was found that the rate of NAFLD was 51 (27.3%), 24 (25.8%), 21 (45.7%), and 9 (56.3%) in control, single, double, and multi lesion groups, respectively, and the difference was statistically significant (P = 0.008). Therefore, the results of this study imply that the number of coronary artery stenosis is associated with the presence of NAFLD, with a higher ratio observed in participants with more severe CAC, as shown in Table 2.
| Control | Single-Vessel | Two-Vessel | Multi-Vessel | P-Value | |
|---|---|---|---|---|---|
| NAFLD (%) | 51 (27.3) | 24 (25.8) | 21 (45.7) | 9 (56.3) | 0.008 |
4.3. Association of NAFLD Severity with the Severity of Coronary Artery Stenosis and the Number of Diseased Vessels
Patients were grouped based on FIB4 score as control (n = 237), mild (n = 49), moderate (n = 48), and severe (n = 8) NAFLD risk. It was found that the stages of NAFLD significantly increased with augmentation in the Gensini score (P = 0.01). Furthermore, a significant increase was noted in the number of diseased vessels among participants in the mild, moderate, and severe groups, compared to participants in the control group (P = 0.001), as shown in Table 3.
a Post-hoc analysis was performed using the LSD method in all the above analyses. P < 0.05 was considered statistically significant.
b P = 0.013
c P = 0.019 in LSD post-hoc comparisons (vs. the control group)
d P < 0.001 in LSD post-hoc comparisons (vs. the control group)
Results of the present investigation indicated that the Gensini score in moderate NAFLD and severe NAFLD groups was higher than in the control group (P = 0.013 and 0.019) as indicated by the LSD post-hoc comparisons. The number of the diseased vessels of participants in the severe NAFLD group was higher than in the control group (P < 0.001). We also conducted univariate logistic regression analyses to examine the association of CAC with FIB4 scores, while it was evident that there was no statistical significance (P = 0.191), as demonstrated in Table 4.
| Variables | OR Value | 95% CI of OR Value | P-Value |
|---|---|---|---|
| Age | 1.058 | 1.030 - 1.086 | < 0.001 |
| Gender | 2.102 | 1.329 - 3.323 | 0.001 |
| Diabetes | 2.017 | 1.026 - 3.965 | 0.042 |
| GLU | - | - | 0.307 |
| TG | - | - | 0.687 |
| HDL | - | - | 0.46 |
| UA | - | - | 0.661 |
| Hypertension | - | - | 0.246 |
| FIB4 index (0) | - | - | 0.093 |
| FIB4 index (mild) | - | - | 0.37 |
| FIB4 index (moderate) | - | - | 0.051 |
| FIB4 index (severe) | - | - | 0.191 |
5. Discussion
The current study evaluated the association between NAFLD and the severity and the extent of coronary stenotic lesions calculated using the Gensini score. It was found that patients with NAFLD had higher Gensini score than the participants without NAFLD. It was evident that the higher the number of patients with coronary lesions, the higher the rates of NAFLD. Moreover, it was evident that the Gensini score and the number of diseased vessels rose with an increase in FIB4 score for patients with NAFLD.
The NAFLD is now recognized as a hepatic component of metabolic syndrome, and this syndrome is associated with the presence and progression of CAC (22). According to the previous studies, NAFLD contributes to an increased CHD morbidity and mortality risk (11, 18, 23-25). Some studies have shown that NAFLD is the common metabolic risk factor for CHD independent of established cardiovascular disease risk factors (26, 27). Mirbagheri et al. revealed a close association between NAFLD and CHD risk after correction for traditional risk factors (28). Another study in Taiwan showed that NAFLD was an independent factor for CAD (moderate to high risk, CACS > 100) besides the normal risk factors, including older age, male gender, and DM (29).
Many studies have indicated that NAFLD is a significant risk factor for an increased problem of cardiovascular events (7, 16, 30). According to Lee et al., the US-determined NAFLD severity positively correlates with CHD risk (18). Song et al. showed that high-grade NAFLD might more commonly predict symptomatic CHD. In a separate study, it has been found that NAFLD and a higher degree of liver stiffness are associated with a higher risk of CHD (31). The prevalence of CHD is higher in patients with moderate to severe steatosis than in patients with mild steatosis (16). The findings of the current research are consistent with the results reported by other investigations.
It is worthy of attention to the patients with NAFLD who have the increased risk of CHD morbidity and mortality. Furthermore, several studies have shown that NAFLD increases CAC prevalence (5, 29, 30). However, to the best of our knowledge, some investigations use computed tomography angiography to diagnose CAC, while in the current study, all patients underwent coronary angiography. It was also found that NAFLD significantly correlates with CAC and Gensini score. Although liver biopsy is the gold standard for NAFLD, it is hardly available in clinical settings. The liver fat score, which identifies people with NAFLD as a non-invasive marker, is associated with CHD and increased cardiovascular risk (32).
The present study used FIB4 scores to predict the severity of NAFLD, which has been confirmed as the highest clinical diagnostic rule and non-invasive method for diagnosing liver fibrosis in patients with NAFLD (21). In the current study, the Gensini score and the number of diseased vessels in patients with severe NAFLD were significantly higher than those in the non-NAFLD group. Therefore, higher liver stiffness is associated with the development risk and progression of CAC. Several studies reported that NAFLD is associated with CAC independent of traditional risk factors (11, 33, 34). We conducted univariate logistic regression analyses to examine the association of the Gensini score with the FIB4 score. However, no statistical significance was found. This could be due to the relatively small sample size or subjects only from one hospital.
The present study had several limitations. First, it was a cross-sectional study at a single center. Therefore, the findings of the current investigation may need further validation using large samples. Second, the diagnosis of NAFLD using ultrasound could have inter-observer variability because different radiologists examined the images. To overcome this problem, two professional radiologists diagnosed all ultrasound images in the present study. Third, we obtained only one measurement in each participant, and the variations were not considered. However, this study was meaningful in that all patients underwent coronary angiography. Furthermore, it was the first to evaluate the relationship between FIB4 score-predicted NAFLD severity and coronary stenotic lesions calculated by Gensini score.
In conclusion, the present research showed a positive relationship between NAFLD severity and CAC in the eastern Chinese population. It was evident that the higher the degree of FIB4 score, the higher the risk of CAC in patients with NAFLD. Notably, the NAFLD severity assessed by the FIB4 score may be useful for differentiating patients who have a higher risk of CAC. However, there is a need for further prospective studies to validate the reported results.