1. Context
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are the most common causes of chronic liver diseases worldwide, including liver cirrhosis, end-stage liver disease, and hepatocellular carcinoma (HCC). It is estimated that approximately 240 - 350 million people are infected with HBV and 71 - 115 million with HCV (1, 2). HBV and HCV share the same modes of transmission and HBV/HCV co-infection is a frequent occurrence, especially in areas endemic for both viruses and among subjects with high risk of parenteral infections. The number of patients with HBV/HCV co-infection is unknown. Testing for HBsAg alone may substantially underestimate the true prevalence of HBV/HCV co-infection with the phenomenon of silent/occult HBV infection (3). The rates of HCV co-infection in HBsAg positive patients vary from 9 to 30%, depending on geographic region (4). In Yu et al. study, 8.4% of patients with chronic HCV infection were co-infected with HBV (5).
The analysis of data from national veterans affairs clinical case registry revealed that among 168,239 patients with HCV exposure, 58,415 patients had also HBV exposure giving the prevalence of 34.7%, and among 102,971 patients with HCV infection, 1,431 patients had also HBV co-infection giving the prevalence of 1.4% (6). In Bini et al. observations, 61.5% of 1,257 patients with chronic HCV infection had evidence of prior exposure to HBV, whereas 5.8% had dual infection with HBV (7). The incidence of the presence of isolated anti-Hepatitis B core antibodies (HBcAb) in HCV infected patients may be higher because screening programs require often only test for HBsAg (3, 7). According to Wang et al. study, the prevalence of occult HBV infection (OBI) defined as the presence of HBV DNA in the absence of HBsAg has been estimated from 11.9 to 44.4% of HCV infected cases (8).
Recently, predictors or risk factors of HBV co-infection in HCV infected patients have been identified. In Tyson et al. study, multivariable logistic regression revealed age less than 50 years, male sex, HIV infection, history of hemophilia and thalassemia, history of blood transfusion, and cocaine use as independent risk factors of HBV co-infection (6). In Bini et al. study, among the independent risk factors for HBV/HCV dual infection, there were age less than 40 years, Asian ethnicity, intravenous drug use, and a greater number of sexual partners (7).
Several experimental and clinical studies have investigated the interactions between both HBV and HCV viruses. It was revealed that HBV and HCV are capable of suppressing replication of one another and HCV infection can suppress HBV replication (7, 9). In patients co-infected with HBV and HCV, most often lower HBV DNA levels and decreased hepatic expression of HBsAg and HBcAg compared to mono-infection with HBV were observed. It was the basis for the hypothesis that there are reciprocal interactions between HBV and HCV, where HBV replication is inhibited by HCV (10).
Many clinical studies have shown that HBV/HCV co-infection compared to HBV or HCV infection alone is associated with faster progression of hepatic fibrosis, more severe liver disease with higher risk of liver cirrhosis, hepatic decompensation, and HCC. In Bini et al. study, comparing HBV/HCV co-infected patients with HCV-mono-infected patients, higher ALT activity, higher necro-inflammatory score, more advanced fibrosis with faster fibrosis rate, and more severe steatosis were observed in histopathological liver pictures of patients with co-infection (5). Since patients with HBV/HCV co-infection are at high risk of liver cirrhosis and HCC, they should be given priority in qualification to antiviral treatment.
2. Evidence Acquisition
To find relevant studies reporting HBV reactivation in HBV/HCV co-infection during anti-HCV therapies, a comprehensive search was conducted on all peer-reviewed journals indexed in PubMed, Scopus, and Web of Science databases. Papers were considered using the appropriate combinations of the following key terms: HBV/HCV co-infection, HBV reactivation, direct-acting antiviral, and anti-HCV therapy.
Furthermore, latest HCV management guidelines from the AASLD/IDSA and European Medicines agency (EMA) recommendations were added to compare the obtained findings and support the importance of reactivation of HBV during anti-HCV treatment.
3. Results
3.1. HBV Reactivation
Reactivation of hepatitis B is defined as an abrupt reappearance or rise of HBV DNA serum level in patients with previously resolved or inactive HBV infection. Reactivation of HBV infection in HBsAg positive patients treated with immunosuppressive or cytotoxic treatment is well known (11-13). Reactivation in patients with resolved HBV infection (HBsAg negative, anti-HBc positive) can also occur during and after immunosuppressive or cytotoxic therapy. According to the hypothesis of reciprocal interactions between HBV and HCV and suppression HBV replication by HCV, the risk of HBV reactivation following successful HCV treatment seems to be important.
3.2. HBV Reactivation During IFN-Based Therapy for HCV
Because IFN can suppress both HBV and HCV replication, HBV reactivation occurs rarely during IFN-based anti-HCV therapies. However, HBV reactivation has been reported during and after treatment with Pegylated Interferon plus Ribavirin (PegIFN+RBV) in patients chronically infected with HBV and HCV. In one report, the reappearance of HBV DNA during treatment with PegIFN + RBV was present in 38% of HBV/HCV co-infected patients with pretreatment serum HBV DNA < 200 IU/mL. None of the recurrent HBV DNA replications was associated with hepatitis flare and none of patients received anti-HBV therapy. In the described study, no clinical hepatitis B developed, and in 30% of patients, the HBsAg seroconversion was observed. The results of this study suggest that interferon-based therapy may suppress replication of both HCV and HBV viruses (14).
Vigano et al. assessed the risk of HBV reactivation in 22 HBV carriers co-infected with HCV treated with IFN and ribavirin (15). During the treatment, serum HBVDNA, HCV RNA, and ALT activity were evaluated every 3-6 months. Sustained HCV viral response was observed in 9/22 patients, and an increase of serum HBV DNA without ALT activity increase was observed in 3 patients. The authors concluded that the risk of HBV reactivation during and after combined (IFN + RBV) treatment was low.
In a meta-analysis of five trials involving 705 Asian patients, performed by Liu et al. to comparatively analyze the response to interferon plus ribavirin treatment in patients with HBV/HCV co-infection and HCV mono-infection, end-of-treatment virological response (ETVR), sustained virological response (SVR), and ALT normalization rate were assessed (9). The rates of ETVR and SVR in both groups of patients were comparable. At the end of follow-up, ALT normalization rates were significantly higher in patients with HCV mono-infection than in patients with HBV/HCV co-infection (P = 0.001). Serum HBV DNA levels and the rate of HBV DNA resurgence in HBV/HCV co-infected patients were significantly higher in patients achieving SVR compared to those treated without SVR (P = 0.009).
Wahle et al. evaluated the HBV reactivation after HCV treatment in 10 hemodialysis patients with HBV/HCV co-infection (HBV DNA lower than 2,000 IU/mL) treated with interferon-alpha and followed them up at least 36 months after HCV treatment (16). HBV reactivation during follow-up was observed in 6/10 patients including 5/6 patients with SVR. Patients with HBV reactivation received lamivudine. The authors concluded that treatment of hepatitis C in HBV/HCV co-infected hemodialysis patients may favor HBV reactivation and therefore, they recommended monitoring of HBV viremia and implementation of anti-HBV therapy.
In Chen et al. meta-analysis, the pooled incidence rate of HBV reactivation among 779 CHC patients with overt HBV was similar among patients treated with IFN-based therapy (14.5%) and those treated with DAAs (12.2%) although there was more clinically significant hepatitis due to HBV reactivation in those treated with pan-oral DAAs compared to those treated with interferon-based therapy. HBV reactivation occurred much earlier in patients treated with IFN-free DAA-based therapy (mostly between 4 - 12 weeks during treatment) compared to those treated with IFN-based therapy (after the end of follow-up) (17).
3.3. HBV Reactivation During IFN-Free Therapy for HCV
The experiences with treatment of chronic hepatitis C with IFN-free direct-acting antiviral (DAA) therapies in patients with HBV/HCV co-infection are very limited because of the exclusion criteria of many clinical studies.
In real-life treatment, some causes of HBV reactivation during treatment with various DAA regimens, including simeprevir plus sofosbuvir (with or without ribavirin), daclatasvir plus asunaprevir, and ledipasvir/sofosbuvir were described (10, 18-20).
Collins et al. presented 2 cases of HBV reactivation during treatment with simeprevir and sofosbuvir (18). The first case concerned a 55-year-old man with history of chronic HBV and genotype 1a HCV co-infection, qualified for IFN-free HCV re-therapy after two previous therapies with peg-IFN and ribavirin. The patient had compensated cirrhosis with Child-Pugh class A. Pretreatment HCV RNA was 1.3 million IU/mL. HBV DNA - 2,300 IU/mL, ALT- 62 IU/mL, and the presence of hepatitis B e antibody (anti-HBe) in his serum were detected. Treatment with sofosbuvir and simeprevir resulted in a rapid decline in HCV RNA to undetectable values at week 4. After 7 weeks of treatment, the patient began malaise, nausea, and abdominal pain with jaundice and hepatomegaly. ALT activity increased to 1,495 IU/L, total bilirubin level to 12.2 mg/dL, INR to 1.96, and HBV DNA to 22 million IU/mL. HCV treatment was discontinued at week 8 and treatment with tenofovir and emtricitabine was initiated. By week 14, the patient’s symptoms resolved and results of LFTs returned to baseline. At week 28, the HCV RNA remained undetectable, and HBV DNA decreased to a level less than 20 IU/mL. Tenofovir and emtricitabine were continued for ongoing HBV suppression.
The second case described by Collins et al. presented a 57-year-old man with history of chronic genotype 1a HCV infection qualified for IFN-free HCV re-therapy (18). Before IFN-free therapy, the HCV RNA serum level was 8.6 million IU/mL, HBV DNA was below the lower limit of quantification (20 IU/mL), and ALT activity was 54 IU/L. HBV serologic studies revealed the serum presence of HBcAb and absence of HBsAg and HBsAb. During treatment with sofosbuvir and simeprevir, serum levels of both HBV DNA and HCV RNA were monitored at 2-week intervals. At week 2, serum level of HCV RNA was undetectable and HBV DNA increased to the value of 353 IU/mL. Because of systematic increase of HBV DNA serum level (1,125 IU/mL at week 4), after 4 weeks of treatment, despite normal liver function tests (LFTs) and asymptomatic duration of the disease, tenofovir was added for treatment of HBV reactivation. Serum HCV RNA level remained undetectable and the HCV and HBV viral loads were also undetectable after 12 weeks of therapy. In both patients presented by Collins et al., DAAs therapy led to undetectable HCV RNA after 2 weeks of therapy. Parallel to HCV clearance, significant increases in HBV replication were observed. Both patients had been previously treated with peg-IFN-α and ribavirin without any signs of HBV reactivation. The rapid increase in HBV replication during treatment with sofosbuvir and simeprevir suggests that the risk of HBV reactivation and acute hepatitis may be greater with newer HCV treatment regimens that may concern patients with positive HBcAb but negative HBsAg and HBsAb. The authors explained that the risk of HBV reactivation may be greater with newer HCV treatment regimens (DAA) compared to PegIFN + RBV because of their higher potency against HCV and lack of anti-HBV activity.
Takayama et al. described HBV reactivation in a 69-year-old male with chronic hepatitis HBV/HCV etiology treated with daclatasvir and asunaprevir (20). Before treatment, the serum ALT activity was 94 U/L, HCV RNA serum level was 4.2 log IU/mL, and HBV DNA serum level was 2.5 log copies/mL. During treatment, ALT activity increased to 237 U/L on day 43 in spite of undetectable HCV RNA. Serum HBV DNA increased to 7.0 log copies/mL at the same time. The treatment was stopped due to suspicion of drug-induced liver injury and/or HBV reactivation. Administration of entecavir could reduce HBV DNA serum levels, followed by a decrease in ALT activity. The authors concluded that close monitoring of HBV DNA during anti-HCV DAA therapy and anti-HBV therapy after increased HBV DNA should be considered in patients with HBV/HCV co-infection.
De Monte et al. reported a case of early HBV reactivation during DAAs-based anti-HCV treatment (ledipasvir/sofosbuvir) in a patient with resolved HBV, HCV genotype 4d, and HIV infection (19). The 53-year-old man with controlled HIV infection with antiretroviral therapy achieved HCV RNA below 12 IU/mL at week 8 of IFN-free therapy of chronic hepatitis C. When treatment finished, dizziness, fever, jaundice, and increased ALT activity (1,026 U/L) and bilirubin level (174 umol/L) were observed. Serum HBV DNA undetectable at the beginning of therapy increased to 8.9 log10 IU/mL. Introduction of tenofovir induced a decrease in HBV DNA while HCV RNA and HIV RNA were still undetectable. In the author’s point of view, the described case highlights the necessity for close monitoring of HBV infection markers in all patients qualified for HCV DAA therapy.
Ou et al. described hepatitis B reactivation in a 53-year-old man after four weeks of therapy of chronic hepatitis C with ledipasvir/sofosbuvir (21). Serum HCV RNA was undetectable after 3 weeks of IFN-free therapy. At the fourth week, the patient appeared weak, had poor appetite and yellow urine. HBV DNA undetectable at the beginning of treatment increased to 8.26E7 IU/mL, ALT activity increased to 779 U/L, and bilirubin to 90.8 umol/L. The patient received entecavir and showed undetectable HBV DNA after five weeks. The authors proposed simultaneous anti-HBV treatment as a more reasonable option for patients with HBV/HCV co-infection treated with DAAs.
The first report of hepatitis B reactivation leading to fulminant hepatic failure and liver transplantation after initiation of IFN-free treatment for hepatitis C was described by Ende et al. (10). The case concerned a 59-year-old woman with chronic hepatitis C genotype 1b and isolated anti-HBc serum presence, who initiated treatment with simeprevir, sofosbuvir, and ribavirin. She responded very well to treatment with near normalization of ALT, and HCV RNA decreased below the level of quantification after 4 weeks of treatment. At week 11 of the planned 12-week course, she developed fulminant hepatic failure due to hepatitis B reactivation and ultimately required liver transplantation.
Garcia et al. presented the case of a 53-year-old patient co-infected with HIV/HCV who developed acute liver failure and died of HBV reactivation during IFN-free therapy of chronic hepatitis C with sofosbuvir and ledipasvir (22). Two years earlier, this patient had been treated with pegylated interferon and ribavirin but the therapy was interrupted due to bad tolerance of PegIFN. During DAAs treatment, serum HCV RNA decreased to less than 15 IU/mL in week 8, and the patient achieved SVR in week 4 of follow-up. A month later, the patient demonstrated abdominal pain, nausea, and jaundice with increased ALT activity to 1025 IU/L, bilirubin serum level to 10.98 mg/dL, and appearance of HBV DNA. Treatment with entecavir was initiated; however, clinical and laboratory evaluations did not confirm ascites and encephalopathy, and the patient died of acute liver failure.
Yeh et al. evaluated 64 patients with chronic hepatitis C co-infected with HBV, including 7 with current HBV infection and 57 with past HBV infection, treated with pan - oral DAA therapy. Compared to patients with past HBV infection, patients with current HBV infection had a significantly lower pre-treatment HCV RNA level (P = 0.016) and higher rate of HBV DNA (P = 0.001). The overall SVR rate was 96.9% and 2 patients - one from each group - had a relapse. HBV virological reactivation was observed in 4 out of 7 HBsAg positive patients. Clinical reactivation was observed in one patient with detectable HBV DNA before treatment that recovered after entecavir administration. No HBV virological reactivation was observed in patients with past HBV infection (23).
These cases raise concern for the risk of severe hepatitis B reactivation in hepatitis B and C-co-infected patients or chronic hepatitis C-infected patients with isolated hepatitis B core antibody treated with DAAs for hepatitis C. HBV reactivation may occur irrespective of the HCV genotype or the class of used DAAs. Presented observations suggest that HBV infection should be monitored parallel to DAAs treatment initiation.
Sulkowski et al. found no evidence of HBV reactivation in cohort of 103 patients with HBcAb serum presence examined at post treatment week 24 of therapy with sofosbuvir-ledipasvir against HCV infection (24). The authors suggested that HBV reactivation in co-infected patients with HBcAb without HBsAg is uncommon.
Gane et al. evaluated whether ledipasvir and sofosbuvir therapy could suppress HCV infection in patients co-infected with HBV (25). All eight patients reached HCV RNA < 15 IU/mL 12 weeks after treatment. In seven of eight patients with SVR, serum HBV DNA levels increased during treatment, but none of the increases was greater than 20,000 IU/mL, and none was associated with clinical HBV flares or required treatment.
In the US food and drug administration (FDA) adverse events reporting system (FAERS) database, 29 cases of HBV reactivation were registered between November 2013 and October 2016 in patients with inactive or resolved HBV infection receiving direct-acting antiviral (DAA) therapy for HCV infection (26). All cases were temporarily associated with DAA initiation. Most of the cases occurred within 4 - 8 weeks of initiation. HBV reactivation resulted in significant clinical complications including liver functions decompensation in 3 patients, leading to death in 2 patients and liver transplantation in one patient. Initiation of HBV treatment was delayed in 7 of 16 (44%) treated cases. Most patients treated for HBV experienced decreased HBV DNA and improved liver function and clinical symptoms.
3.4. Risk Factors for HBV Reactivation
Wang et al. performed an observational study to determine the incidence and factors associated with hepatitis in 327 patients receiving DAAs agents for HCV infections in areas endemic for HBV in China (8). Among the examined patients, 10 were positive for HBsAg, and 124 had occult HBV infection. In the total study population, 10 patients (3.1%) had hepatitis. In 3 cases, hepatitis was associated with HBV reactivation including 1 case not in the icteric phase, 1 case in the icteric phase, and 1 case with liver failure. The strong risk factor for developing hepatitis during treatment was the presence of HBsAg before DAA treatment (P < 0.001). However, based on the recorded cases, FDA suggests that HBV reactivation is independent of HCV genotype, DAA use, and baseline HBV disease parameters. Up to now, the mechanism by which HCV-therapy might cause HBV reactivation is not known and there are no genetic markers for prediction of HBV reactivation (26).
3.5. Recommendations
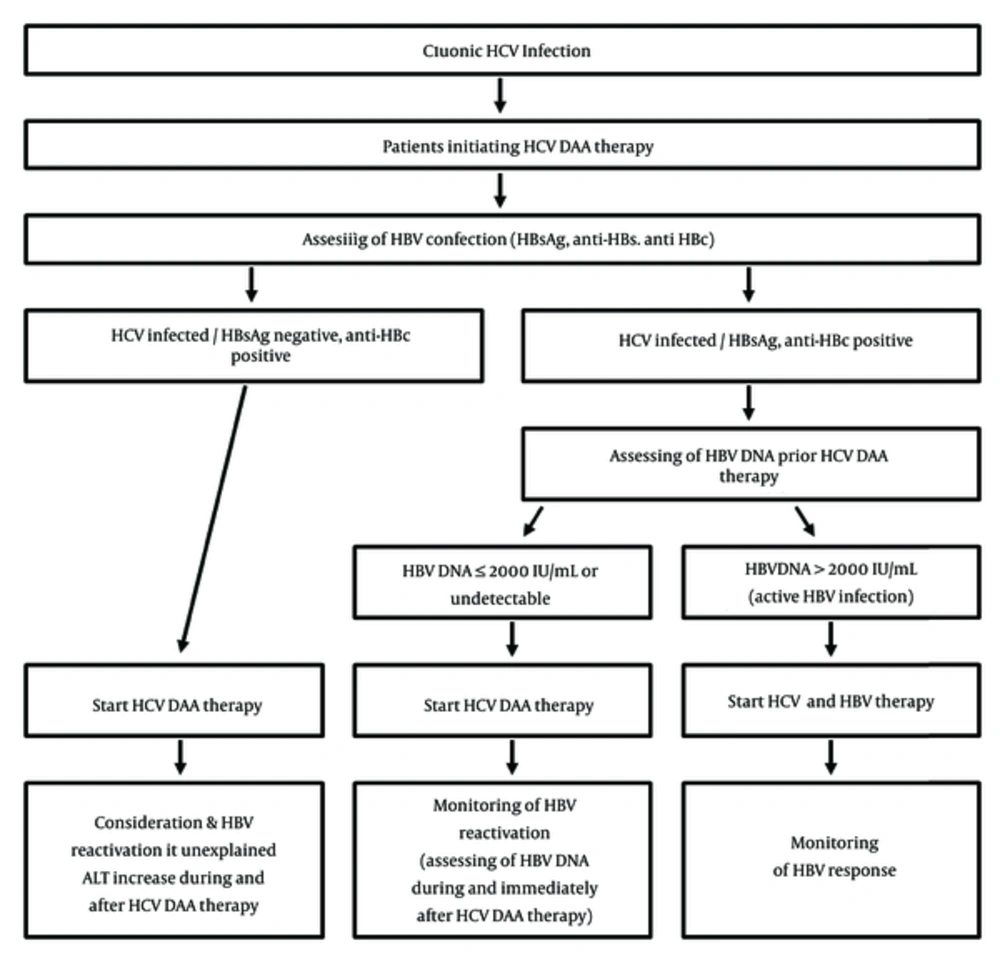
Presented data supports the idea that effective elimination of HCV may increase the risk of the shift in immunological control. In part because of this, the latest HCV management guidelines from the AASLD/IDSA now recommend that all patients starting HCV DAA therapy should be assessed for HBV co-infection with HBsAg, anti-HBs, and anti-HBc testing (27). For HBsAg positive patients, the test for HBV DNA should be obtained and patients meeting criteria for HBV treatment should start therapy at the same time or before HCV DAA therapy initiation. According to the same recommendations, patients with low or undetectable HBV DNA levels should be monitored at regular intervals for HBV reactivation. For patients with the presence of anti-HBc alone or for anti-HBs and anti-HBc, the possibility of HBV reactivation should be considered in case of an increase in LFTs during or after DAAs treatment (Figure 1).
On December 2, 2016, the Pharmacovigilance risk assessment committee (PRAC) of the European Medicines agency (EMA) issued a news release confirming the risk of HBV reactivation in patients being treated with DAA agents for HCV (28). On December 16, 2016, the European Medicines agency (EMA) confirmed its recommendation to screen all patients for hepatitis B before starting treatment with direct-acting antivirals for hepatitis C. Patients infected with HCV and HBV must be monitored and managed according to current clinical guidelines (29).
4. Conclusions
It seems close monitoring of HBV co-infection by HBV DNA, HBsAg, anti-HBc, and ALT assessment may be warranted during any anti-HCV therapy regardless of the used DAAs class or the HCV genotype. Anti-HBV therapy with nucleoside analogs parallel or after the increase of HBV DNA should be considered in patients with HBV/HCV co-infection.