1. Background
Mesenchymal stem cells (MSCs) are the most promising tools for cell treatment and human tissue regeneration because of their multi-potency of differentiation, self-renewal capability, paracrine effects, and immune-modulatory potential (1). Mesenchymal stem cells are isolated from bone marrow (BM), adipose tissue (AT), cord blood, and Wharton’s jelly (WJ-MSCs). Due to limited proliferative capacity, low cell concentration, invasive and painful isolation process, and the risk of infection, the therapeutic use of adult MSCs, such as BM, has been hampered. Instead, WJ-MSCs have much potential for allogeneic and autologous transplantation because of their high availability, low cost, and ease, non-invasive isolation method. Also, WJ-MSCs have a high proliferative capacity, are non-tumorigenic, and induce no teratoma after transplantation. As a result, WJ-MSCs are excellent for regenerative medicine (2). Mesenchymal stem cells repair tissue damage through paracrine mediators such as exosomes (3).
Exosomes are one of the MSC-derived paracrine mediators (4). They are small membrane-bound vesicles ranging from 30 to 100 nm secreted by different cell types. They also carry cargos such as proteins, mRNA, and non-coding RNA to target tissues (5).
Many studies demonstrated that MSCs have immunomodulatory functions linked to several factors and molecules secreted by these cells (6). The immunomodulatory functions of MSCs may depend mainly on their microenvironment in tissues. Indeed, the types and concentration of inflammatory mediators in MSCs microenvironment can affect MSCs’ function and therapeutic potency (7). Pretreatment with growth factors, including cytokines, can alter host tissue via autocrine and paracrine pathways, resulting in downstream signal transduction for cell differentiation and survival.
Mesenchymal stem cells have a variety of cytokines and growth factors with different biological functions, such as anti-inflammatory and immune-modulatory effects. However, their short biological half-life inhibits their permanent therapeutic effects (8). Pretreatment of MSCs with pro-inflammatory mediators is commonly used to mediate inflammation in damaged tissues (9), reduce oxidative stress and apoptosis (10), improve MSCs efficacy in vivo (1), and enhance allotransplant survival and function (11). Several studies show that the pretreatment of MSCs is effective in repairing various injured tissues, including diabetes mellitus (12), acute lung injury (ALI) (13, 14), nephropathy (15), and induced radiation damage (16).
One of the influential immunosuppressive cytokines often presenting in inflammatory states is the transforming growth factor-beta 1 (TGFβ1) (17). The TGFβ pathway is believed to play a dual role during the pathogenesis of inflammation depending on its concentration and participating cell types (18). Mesenchymal stem cells have receptors for TGFβ to respond to it (19). Although TGFβ is an immunosuppressant (20), its high concentrations during the inflammatory process may intensify MSCs’ inflammatory responses (19).
Many basic and clinical studies have demonstrated the beneficial effects of MSC-based therapy on liver fibrosis (21). The immunomodulating activity of MSCs makes them very effective for regulating the inflammatory process in liver fibrosis (22). Fibrosis that leads to the over-deposition of extracellular matrix (ECM) proteins is a consequence of chronic injury and inflammation of the liver (23). Following a fibrogenic stimulus, hepatic stellate cells (HSCs) are effectively converted to fibrogenic myofibroblasts (24). In liver fibrosis, ECM accumulates excessively in the liver, leading to cirrhosis and hepatocellular carcinoma (HCC) (25). Transforming growth factor-beta is one of the crucial pro-inflammatory and profibrogenic mediators in liver fibrosis (26), which plays an essential role in regulating critical biological phenomena through the TGFβ signaling pathway in MSCs (27), including proliferation (28), differentiation (29), and migration of MSCs (30). The TGFβ pathway is believed to play a dual role in the pathogenesis of inflammation depending on its concentration and participating cell types (18). The secretory level of TGFβ1 is elevated in injured areas, which promotes the repair of damaged areas (31). Transforming growth factor-beta, through the activation of HSCs, promotes excessive expression and accumulation of ECM proteins in a Smad2/3-dependent pathway (32). Therefore, HSCs inactivation has emerged as an important therapeutic target to regress liver fibrosis (33). However, the optimization of these external agents for the pretreatment of MSCs and the underlying mechanisms need to be further explored to improve the therapeutic effects of MSCs.
2. Objectives
We evaluated the effects of WJ-MSCs-derived exosomes on fibrotic gene expression, including α-smooth muscle actin (α-SMA), E-cadherin, and collagen1α1 and Smad2/3 phosphorylation. Moreover, we further investigated whether the pretreatment of WJ-MSCs with different concentrations of TGFβ changes the anti-fibrotic properties of their exosomes.
3. Methods
3.1. Materials
Low glucose-Dulbecco’s Modified Eagle’s Medium (DMEM), penicillin-streptomycin, and trypsin were provided from Idea-Zist, and fetal bovine serum (FBS) was provided from Gibco. Phospho-Smad2/3 (Thr8) polyclonal antibody was purchased from Elabscience, and α-SMA (CGA7), CD81 (B-11), E-cadherin (67A4), CD9 (C-4), COL1A1 (3G3), m-IgGκ BP-HRP, mouse anti-rabbit IgG-HRP, and GAPDH (6C5) from Santa Cruz Biotechnology, Inc. Beckman Coulter Company provided FITC-conjugated antibodies for CD34 and CD45 and PE-conjugated antibodies for CD44 and CD105 (Nyon, Switzerland). The recombinant human TGFβ1 protein was provided from Abcam, UK. The TGF-β1 protein was solubilized in 10 mM Citric acid, pH: 3.0, and stored at -80°C per the manufacturer’s recommendation. Adipogenic and osteogenic media were provided from Bonyakhte, Iran. Oil Red O and Alizarin Red S were obtained from Sigma-Aldrich, St. Louis, MO, USA. A bicinchoninic acid assay (BCA) protein assay kit was purchased from Parstous Biotechnology, Iran, enhanced chemiluminescence (ECL) system from Bio-Rad, USA, and RIPA lysis buffer and protease inhibitor cocktail from Santa Cruz Biotechnology, USA.
3.2. Cell Lines and Culture Conditions
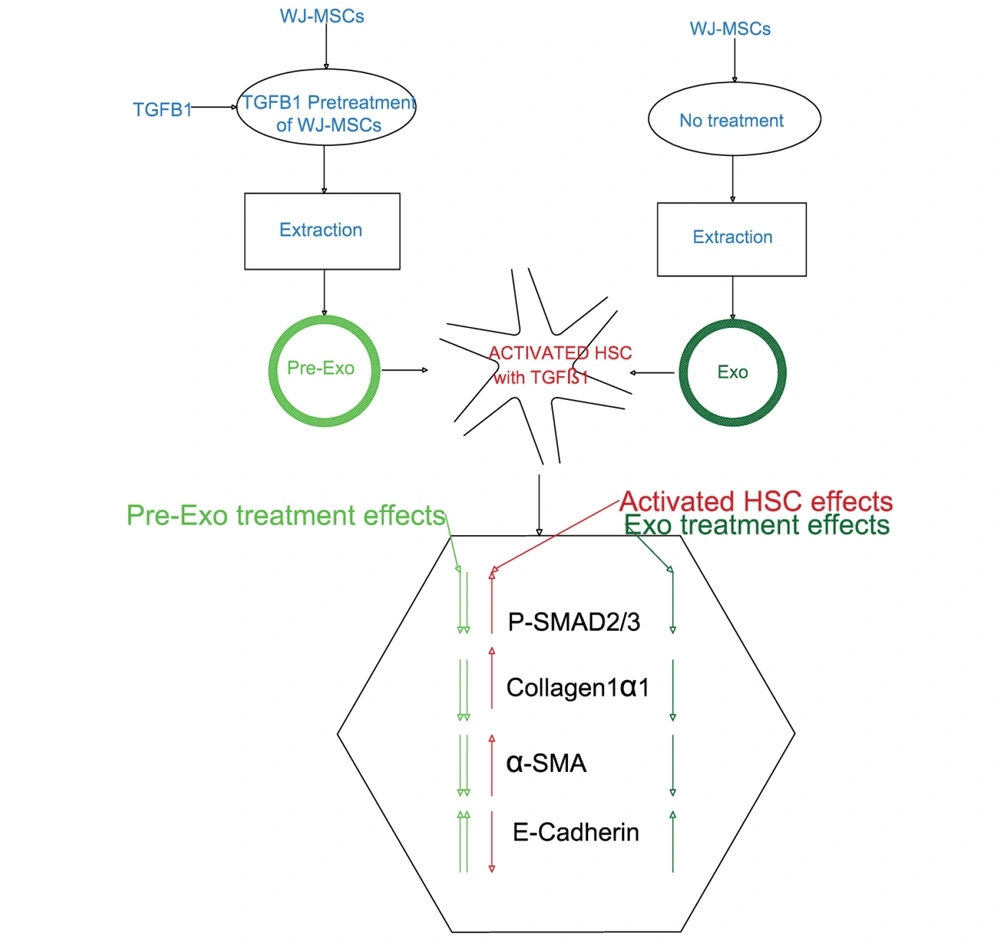
The whole process is schematically shown in Figure 1. In this experimental study, human HSC cell line LX-2 was gifted by Scott L. Friedman, Mount Sinai School of Medicine, New York, NY, USA. LX-2 cells were cultured in a low glucose-DMEM medium with 1% antibiotics penicillin/streptomycin supplemented with 10% FBS in 5% CO2 at 37°C. LX-2 cells were seeded into six-well plates (1 × 104 cells/well) for the in vitro experiment. After attaining 80% confluency, LX-2 cells starved for 16 hours in DMEM containing 0.1% FBS. LX-2 cells were treated with 10 ng/mL TGFβ1 for 72 hours to activate HSCs. After that, the cells were collected and stored at -70°C for subsequent examination.
3.3. Primary Isolation and Identification of Wharton’s Jelly Mesenchymal Stem Cells
After obtaining informed consent from the parents of neonates, WJ-MSCs were isolated from the umbilical cords. The umbilical cord tissue was extensively cleaned and washed with phosphate-buffered saline (PBS) before being transferred to the laboratory under sterile conditions. Then, the umbilical cord tissue was cut into 3 - 5 mm pieces, and its vessels were discarded. Wharton’s jelly mesenchymal stem cells were grown in usual growth media (DMEM supplemented with 10% FBS, 100 U/mL penicillin, and streptomycin) and maintained at 37°C in a 5% CO2 incubator. After three to five days, non-adherent cells were removed. The media were replaced every day until cells reached 80% confluency. Wharton’s jelly mesenchymal stem cells were cultured for three passages and then used for experiments.
The capacity of WJ-MSCs to differentiate into adipogenic and osteogenic lineages was determined. Adipogenic and osteogenic differentiation was induced by WJ-MSC culture for three weeks in adipogenic and osteogenic media, respectively. Adipogenic differentiation was assessed using Oil Red O staining to determine intracellular fat accumulation. Extracellular matrix calcification was investigated by Alizarin Red S using an inverted microscope (Olympus IX50, Japan).
Flowcytometry analysis characterized the phenotypes of WJ-MSCs. It was done by phycoerythrin (PE)-labeled human anti-CD44 and anti-CD105 (as positive markers) and fluorescein isothiocyanate (FITC)-labeled anti-CD34 and anti-CD45 (as negative markers). On a FACSCanto plus instrument (BD Bioscience, San Jose, CA, USA), at least 10,000 events were collected, and the results were analyzed using FlowJo software.
3.4. Transforming Growth Factor-Beta 1 Pretreatment of Wharton’s Jelly Mesenchymal Stem Cells
Recombinant human TGFβ1 dilutions were made from aliquots in serum-free DMEM immediately before use. Different concentrations of TGFβ1 were used to treat WJ-MSCs (0.1, 0.5, 1, 5, and 10 ng/mL) (14). Initially, WJ-MSCs were pretreated with serum-free control media and different concentrations of TGFβ1 (diluted in serum-free media) for 24 h. Afterward, the stimulations were removed, and both control and pretreated cells were cultured in serum-free or specific differentiation induction media to determine the effects of pretreatment on MSC functions.
3.5. Extraction and Identification of Exosome-Derived Wharton’s Jelly Mesenchymal Stem Cell
The exosomes were isolated from the supernatants of WJ-MSC cultures. The WJ-MSCs medium was replaced with a medium containing less FBS to isolate exosomes every two days. Exosomes were isolated from FBS-free conditional medium (CM) using the Exocib kit (Cib Biotech Co.) after the FBS level was gradually reduced to zero. Seventy-two hours later, the CM medium was collected and centrifuged at 4,000 × g for 10 min at +4°C to remove cell debris. Briefly, the filtered CM was combined with the exosome precipitation solution and incubated at +4°C overnight. The samples were centrifuged at 3,000 rpm for 40 minutes. The pellet was then re-suspended in PBS after the supernatants were discarded. Finally, the purified exosome samples were kept at -70°C for storage.
The exosomes were fixed with 4% paraformaldehyde, loaded on carbon grids, and then dyed with 1% phosphotungstic acid at room temperature for two minutes. Transmission electron microscopy (TEM; Zeiss EM10C 100 KV, Germany) was used to investigate the morphological features of isolated exosomes. Dynamic light scattering (DLS) is a technique to determine the size distribution of particles with diameters ranging from 1 nm to 6 μm. Dynamic light scattering Zetasizer Nano ZS was used to determine the sample size distribution (Malvern Instruments, UK). A BCA protein assay kit was used to measure the protein concentration of the exosomes. The identification of exosomes was performed by the Western blot assay using typical exosome markers (CD9 (1: 300; Santa Cruz) and CD81 (1: 100, Santa Cruz)). Briefly, the 20 μg protein samples were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto PVDF membrane, and then incubated with primary antibodies of anti-CD9 and anti-CD81 at 4°C overnight, after blocking with 5% non-fat milk for one hour at 37°C. After three 10-min washes in TBST, the membranes were treated for two hours at 37°C with the mouse anti-rabbit IgG-HRP (1: 1000; Santa Cruz), rinsed again, and incubated with chemiluminescent substrates. An ECL system was used to visualize the blots. The extracted exosomes (50 μg/mL) (34, 35) were added to the plates to treat HSCs, while control cells remained untreated.
3.6. RNA Extraction and qRT‐PCR
Total RNAs were isolated using an RNA extraction kit (Biobasic, Canada). Reverse transcription was conducted with a 5X All-In-One RT-PCR Master Mix kit (Biobasic, Canada) using the ABI Prism Quantistudio3 Sequence Detection System (Applied Biosystems, Foster City, CA). SYBR Green master mix performed the PCRs (amplicon). The average expression of each target gene was demonstrated relative to an internal reference gene (GAPDH). The 2-ΔΔCt method was used to calculate the relative expression of each gene. The gene expressions of α-SMA, E-Cadherin, and collagen1α1 were then investigated after LX2 was treated with TGFβ1 at a concentration of 10 ng/mL. These experiments were performed three times. The primer sequences for investigated genes are listed as follows:
ACTA2 (forward): 5’-TGGCTATTCCTTCGTTACTACTGCT-3’, ACTA2 (reverse): 5’-CATCAGGCAACTCGTAACTCTTCTC-3’, COL1A1 (F): 5’-CCTGGGTTTCAGAGACAACTTC-3’, COL1A1 (R): 5’-TCCACATGCTTTATTCCAGCAATC-3’, CDH1 (F): 5’-TACCCTGGTGGTTCAAGCTG-3’, CDH1 (R): 5’-CCTGACCCTTGTACGTGGTG-3’, GAPDH (F): 5’-ACTTTGGTATCGTGGAAGGACT-3’ and GAPDH (R): 5’-GTAGAGGCAGGGATGATGTTCT-3’.
3.7. Western Blot
The cells were homogenized using RIPA lysis buffer and a protease inhibitor cocktail. Then, it was centrifuged at 14,000 g for 20 minutes at 4°C, and the supernatant was collected. After determining protein concentrations, equal amounts of protein (50 μg) were loaded into a 7.5 - 12% SDS-PAGE gel for electrophoresis. Then, gels were electroblotted onto nitrocellulose membrane, which was blocked for one hour at room temperature with 5% non-fat blocking grade milk (Bio-Rad, USA). Primary antibody staining was performed against E-Cadherin (1: 1000, Santa Cruz), α-SMA (1: 1000, Santa Cruz), Collagen1α1 (1: 1000, Santa Cruz), and phosphorylation Smad2/3 proteins (1: 1000, Elabscience) at 4°C overnight. The procedure was followed by the HRP-linked secondary antibody (1: 1000, Santa Cruz) staining. After washing in TBST, the samples were treated with mouse anti-rabbit IgG-HRP (1: 1000, Santa Cruz) for one hour at room temperature. Blots were observed using an ECL kit, and the bands were detected using Bio-Rad ChemiDoc XRS+.
3.8. Statistical Analysis
PRISM software performed all statistical analyses (Graphpad, San Diego, CA). The between-group comparisons were made using ANOVA, followed by Tukey post hoc tests. The differences were considered statistically significant at P < 0.05. Results are expressed as the mean ± standard error of means (SEM) of at least three independent experiments.
4. Results
4.1. Identification of Phenotype in Wharton’s Jelly Mesenchymal Stem Cell
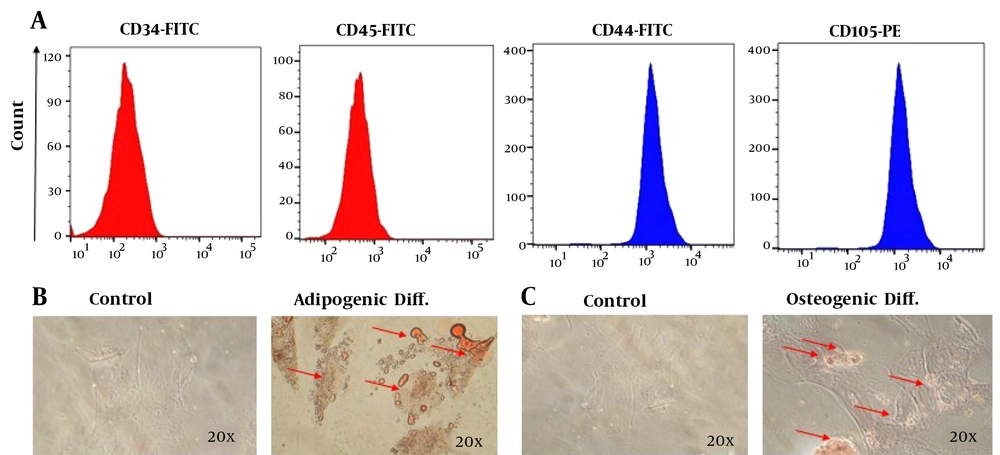
As shown in Figure 2A, flowcytometry revealed that obtained cells were positive for CD105 and CD44 and negative for CD45 and CD34, indicating the characteristics of WJ-MSCs. The cell morphology was fibroblastic, typical of WJ-MSCs. Adipogenic differentiation was confirmed with increased fat accumulation in red granules observed using Oil red O staining (Figure 2B). A significant increase in calcium deposition after staining with Alizarin Red approved the osteogenic differentiation of WJ-MSCs (Figure 2C).
Identification of phenotypes and differentiation of Wharton's jelly mesenchymal stem cells (WJ-MSC). A, Identification of WJ-MSC phenotype. Immunophenotyping of WJ-MSCs by flowcytometry. The cells expressed CD44 and CD105, but not CD34 and CD45; B, adipogenic differentiation potential of mesenchymal stem cells (MSCs). In adipogenic differentiation, lipid droplets were stained with Oil Red O; C, osteogenic differentiation potential of MSCs. In osteogenic differentiation, the mineralized matrix was stained with Alizarin Red S.
4.2. Characterization of Wharton’s Jelly Mesenchymal Stem Cell Exosome
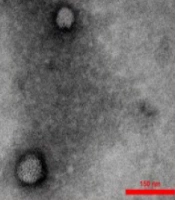
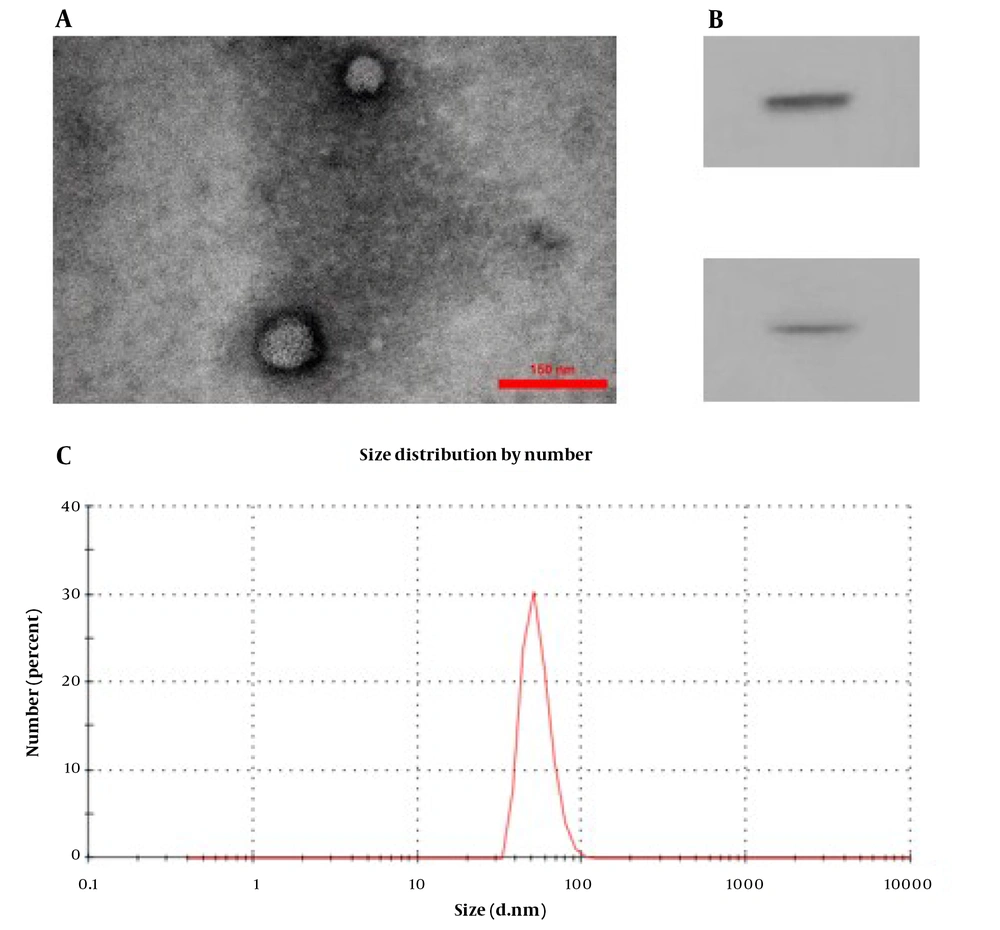
The TEM photograph showed the extracted exosomes of round and elliptical vesicles with a complete double-layer membrane and low electron density materials (Figure 3A). The marker proteins of CD9 and CD81 were expressed in exosomes with significantly higher levels than in WJ-MSCs using western blot analysis (Figure 3B). Finally, the exosome size analysis revealed that they had a mean diameter of approximately 73 nm (Figure 3C). All results indicated the successful isolation of WJ-MSCs and the exosomes of WJ-MSCs.
Characterization of Wharton's jelly mesenchymal stem cell exosome (WJ-MSC-Exo). A, transmission electron microscopy image of WJ-MSC-Exo to visualize the shape and size of these vesicles (scale bar: 0.3 μm); B, the size distribution of WJ-MSC-Exo by Malvern zeta sizer; C, western blot analysis for CD9 and CD81 expression in WJ-MSC-Exo.
4.3. Influence of Transforming Growth Factor-Beta 1 on mRNA Expression and Protein Levels of Extracellular Matrix Components
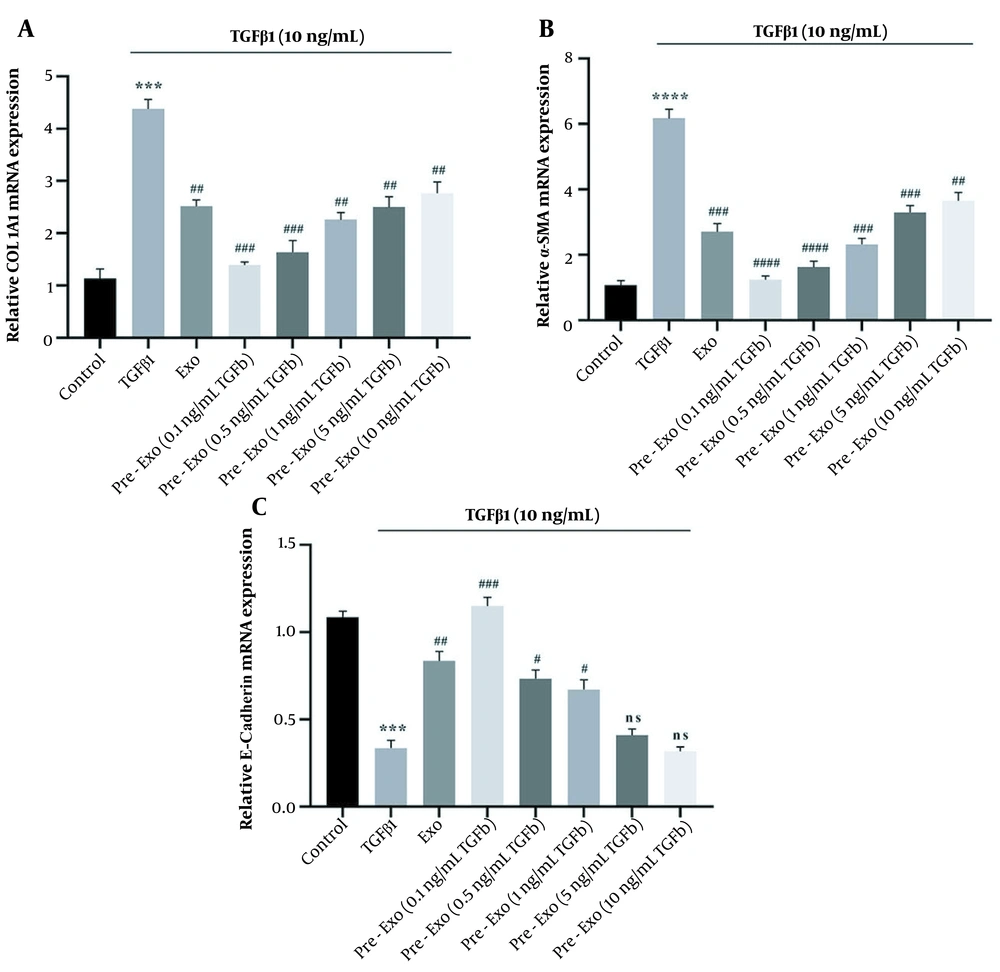
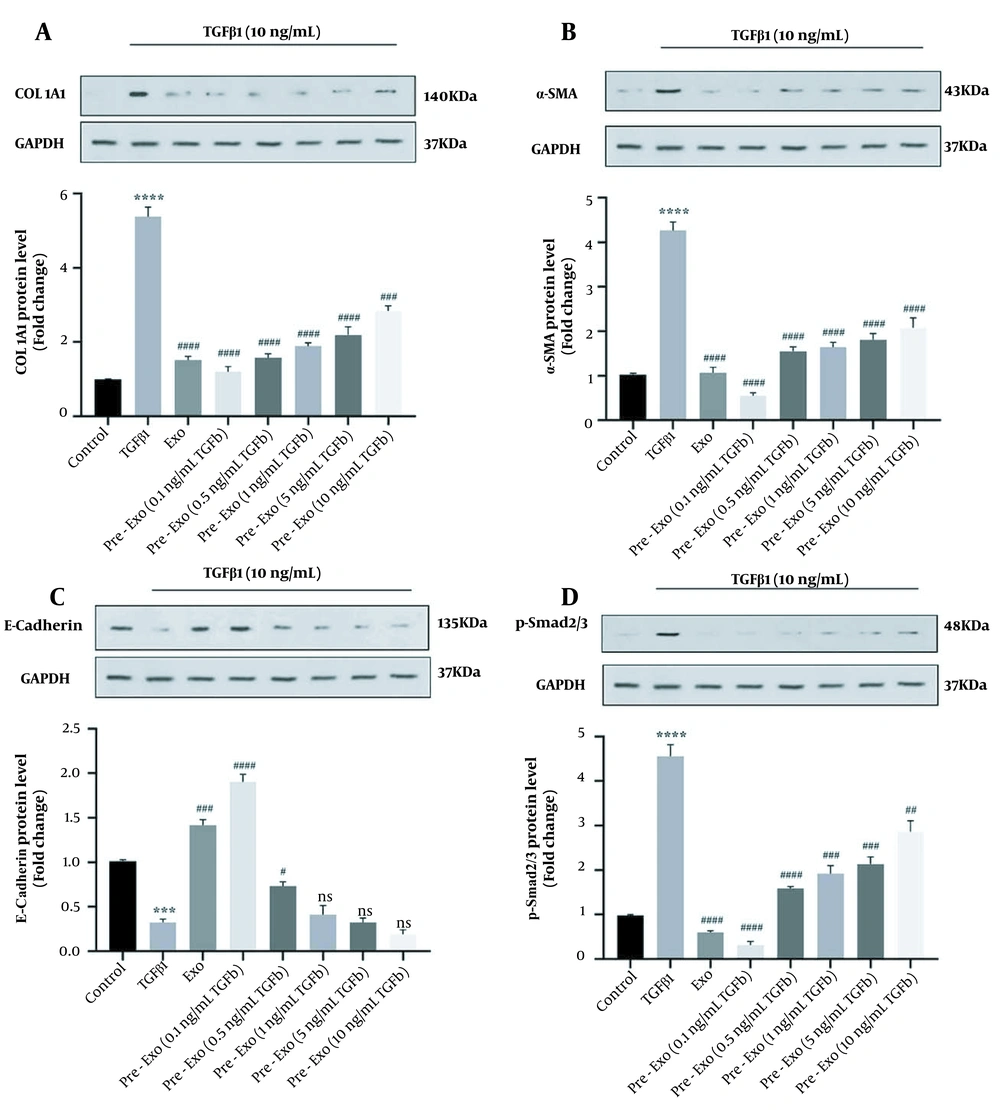
Treatment with TGFβ1 (10 ng/mL) significantly increased the expression level of ECM genes such as collagen1α1 (P < 0.0001) and α-SMA (P < 0.0001) and decreased E-cadherin (P < 0.0001) of activated HSCs (Figure 4A - C). Furthermore, treatment with TGFβ1 increased the protein expression levels of Collagen1α1 (P < 0.0001), α-SMA (P < 0.0001), and p-Smad2/3 (P < 0.0001) and decreased E-cadherin (P = 0.001) compared to the untreated control (Figure 5A - D).
Effect of Wharton's jelly mesenchymal stem cells (WJ-MSCs) (untreated and pretreated) and their exosomes on the expression of fibrotic markers in transforming growth factor-beta 1 (TGFβ1)-activated LX-2 cells using RT-qPCR. The cells in the control group received no treatment; TGFβ1 group: Hepatic stellate cells (HSCs) cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10 ng/mL TGFβ1 for 72 hours, exo group: HSCs cultured in DMEM with 10 ng/mL TGFβ1 for 72 hours, followed by exposure to 50 μg/mL exosomes for 24 hours; pretreated exo group: HSCs cultured in DMEM with 10 ng/mL TGFβ1 for 72 hours, followed by 24 hours exposure to 50 μg/mL of different concentration of exosomes (0.1, 0.5, 1, 5, and 10 ng/mL) derived from TGFβ1-pretreated WJ-MSCs. The relative expression of each gene was normalized to that of controls. All data are shown as means ± standard error of means (SEM). Statistical significance at * P < 0.05, ** P < 0.01, and *** P < 0.001.
4.4. Influence of Transforming Growth Factor-Beta 1-Pretreated Wharton’s Jelly Mesenchymal Stem Cell Exosome on mRNA Expression and Protein Levels of Extracellular Matrix Components in Activated Hepatic Stellate Cells
We used activated HSCs to evaluate the therapeutic effects of TGFβ1-pretreated WJ-MSCs exosomes in vitro. Untreated exosomes and TGFβ1-pretreated exosomes significantly downregulated the p-Smad2/3 level (untreated exosome, 0.1, 0.5, and 1 ng/mL TGFβ1-pretreated exosomes: P < 0.0001, 5 ng/mL TGFβ1-pretreated exosomes: P = 0.0001, and 10 ng/mL TGFβ1-pretreated exosomes: P = 0.0021, Figure 5D). Exposure of activated HSCs to 50 μg/mL exosomes for 24 hours (Exo group) decreased the mRNA expression of α-SMA (P < 0.0001, Figure 4B) and collagen1α1 (P < 0.0001, Figure 4A) and increased the mRNA expression of E-cadherin (P < 0.0001, Figure 4C). A low concentration of TGFβ1-pretreated exosomes of WJ-MSCs (0.1 ng/mL) significantly decreased the expression level of ECM genes (collagen1α1 and α-SMA; P < 0.0001, Figure 4A and B) while significantly increasing E-cadherin expression (P < 0.0001; Figure 4C). Pretreated exosomes of WJ-MSCs with low TGFβ1 concentration (0.1 ng/mL) were more effective than untreated exosomes of WJ-MSCs (P < 0.05). Therefore, TGFβ1-pretreated exosomes of WJ-MSCs (0.1 ng/mL) decreased the ECM proteins by inhibiting TGFβ1/Smad signaling in activated HSCs (Figure 5).
Effect of Wharton's jelly mesenchymal stem cells (WJ-MSCs) (untreated and pretreated) and their exosomes on the expression of fibrotic markers and phosphorylation Smad2/3 in Transforming growth factor-beta 1 (TGFβ1)-activated LX-2 cells using western blot. The cells in the control group received no treatment; TGFβ1 group: Hepatic stellate cells (HSCs) cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10 ng/mL TGFβ1 for 72 hours; exo group: HSCs cultured in DMEM with 10 ng/mL TGFβ1 for 72 hours, followed by exposure to 50 μg/mL exosomes for 24 hours; pretreated exo group: HSCs cultured in DMEM with 10 ng/mL TGFβ1 for 72 hours, followed by 24 hours exposure to 50 μg/mL of different concentrations of exosomes (0.1, 0.5, 1, 5, and 10 ng/mL) derived from TGFβ1-pretreated WJ-MSCs. The relative expression of each gene was normalized to that of control. All data are shown as means ± standard error of means (SEM). Statistical significance at * P < 0.05, ** P < 0.01, and *** P < 0.001.
5. Discussion
Mesenchymal stem cells are activated in vivo by inflammation and in vitro by pretreatment, to acquire the ability to suppress (36). Mesenchymal stem cells do not inherently suppress the immune system but acquire this ability when activated by inflammatory cytokines (37). They also have immunoregulatory plasticity (7). These cells may exert inflammatory or anti-inflammatory functions based on their microenvironments. In liver fibrosis, inflammatory cytokines are present in the microenvironment (19). Depending on type and severity, these cytokines can manipulate the immunoregulatory properties of MSCs (36). One of the crucial cytokines involved in liver fibrosis is TGFβ1 (38). While TGFβ1 is a well-known immunosuppressive cytokine (39), it is also a master driver in the inflammatory and fibrosis processes (40). In liver fibrosis, MSCs’ behavior may change relative to the increasing amount of TGFβ1 (25, 27). These changes consequently affect the communications of MSCs with other cells in the liver, mainly through altering MSC-secreted exosomes (8). Our study demonstrated that WJ-MSCs-derived exosomes ameliorated fibrotic gene expression of activated LX-2 cells. We further delineated that WJ-MSCs pretreatment with low-level TGFβ1 (0.1 ng/mL) significantly intensified the effects of their derived exosomes on fibrotic gene expression of activated LX-2 cells.
The activation of HSCs is the most profound event in hepatic fibrogenesis, and in this event, TGFβ1/Smad signaling is a pivotal player. Inflammatory cytokines, including TGFβ1, through the phosphorylation of transcription factor Smad, upregulate the expression of ECM proteins, including collagen1α1, fibronectin, and α-SMA, leading to liver fibrogenesis (26). Therefore, a therapeutic approach to inhibit TGFβ1/Smad signaling may be an effective strategy for treating hepatic fibrosis (19). Many clinical and basic studies have proven the beneficial effects of MSCs therapy in liver fibrosis (21). In line with these findings, previous studies have shown that treating with human umbilical cord MSCs significantly improved liver function and hepatic fibrosis by inhibiting the paracrine TGFβ1/Smads pathway (41). However, their clinical application is challenging due to storage limitations and cellular senescence during in vitro proliferation (42, 43). Emerging evidence revealed that MSC exosomes exerted the same therapeutic effects as MSCs (44). Exosomes of MSCs show more biological functions than MSCs due to their higher stability and plasma membranes, making them less susceptible to disruption (45). Based on these findings, exosomes are a cell-free treatment that may be safer than direct treatment with MSCs and provide a potential method for tissue regeneration.
Thus, exosomes may be promising candidates for regressing fibrogenic and inflammatory processes (46). Recent studies have shown that priming MSCs with various drugs, cytokines, or growth factors increases the therapeutic efficacy of their exosomes (47). A study by Ghosh et al. showed that pretreatment of BM-MSCs in mice with TGFβ1 resulted in wound closure and healing in a syngeneic mouse wound model (48). In injury sites, TGFβ1 secretion is a critical way to utilize BM-MSCs, and TGFβ1 pretreatment may be an effective way to increase homing and migration of BM-MSCs (31). In this study, we aimed to modify the immunosuppressive abilities of WJ-MSCs exosomes by treating these cells with TGFβ. Our results showed that exosomes derived from untreated WJ-MSCs could regress TGFβ-Smad signaling and expression of fibrotic markers in activated LX-2 cells. However, these effects were significantly profound with applying exosomes derived from 0.1 ng/mL TGFβ-pretreated WJ-MSCs. These results suggest that low levels of TGFβ may increase the anti-fibrotic capacity of WJ-MSCs-derived exosomes. Jiang et al.’s study showed that low concentrations of TGFβ1 in CD18 -/- wounds stimulated the secretion of TGFβ1 from MSCs, increasing myofibroblast differentiation, wound contraction, and vascular formation (49). In contrast, high levels of TGFβ1 may inhibit TGFβ1 production (49).
In this study, the expression level of p-Smad2/3 was reduced in TGFβ1-pretreated exosomes of WJ-MSCs. Also, this study showed that by exposing WJ-MSCs to inflammatory conditions, TGFβ1-pretreated WJ-MSCs-Exo showed more protective effects in activated HSCs than untreated WJ-MSCs-Exo. Lynch et al. showed that pretreatment of mouse MSCs with TGFβ1 significantly modulated the inflammatory phenotype (36). Transforming growth factor-beta 1-pretreated MSCs prolonged graft survival in the mouse model of corneal transplantation. One of the therapeutic effects of MSCs is related to Smad2/3 phosphorylation (36).
In the present study, E-cadherin mRNA expression and protein levels showed the highest upregulation in response to the lowest concentration of TGFβ1-pretreated WJ-MSCs-Exo (0.1 ng/mL) compared to the untreated WJ-MSCs-Exo group. Cadherins mediate cell-cell adhesion (50). This study also demonstrated that a low concentration of TGFβ1-pretreated WJ-MSCs-Exo (0.1 ng/mL) decreased the mRNA and protein expression level of ECM components, including α-SMA and collagen1α1. The RT-PCR results showed that the expression levels of collagen1α1 and α-SMA were decreased by 0.1 ng/mL TGFβ1 in activated HSCs.
However, the action mechanism of low concentrations of TGFβ1-pretreated WJ-MSCs-Exo in activated HSCs has not been fully elucidated. It is hypothesized that TGFβ1-pretreated WJ-MSCs-Exo may act in different signaling pathways in activated HSCs in a concentration-dependent manner. In this study, we observed the dose-response effects of TGFβ on WJ-MSCs-derived exosomes. While WJ-MSCs derived exosomes conditioned with a low concentration of TGFβ (0.1 ng/mL) exerted maximum inhibitory effects on TGFβ-Smad signaling and fibrotic gene expression of activated LX-2 cells, these inhibitory effects became weaker with increasing concentrations. There are discrepant conclusions about the results of TGFβ priming on MSCs. Despite the critical role of TGFβ in the modulation of the immunosuppressive ability of MSCs (27), there are conflicting arguments among researchers. Li et al. demonstrated the immunosuppression potential of TGFβ1 overexpressed MSCs on co-cultured T lymphocytes (51). Conversely, Xu et al. showed that TGFβ could decrease the immunosuppression capacity of MSCs (52). Our findings showed that it might be related to the dose-dependent response of WJ-MSCs to TGFβ. However, it remains challenging to prepare MSCs in vitro and transfer them to a damaged site in vivo with high efficacy (53).
5.1. Conclusions
Exosomes derived from untreated WJ-MSCs could regress TGFβ-Smad3 signaling and expression of fibrotic markers in activated LX-2 cells. However, these effects were significantly profound with applying exosomes derived from 0.1 ng/mL TGFβ-pretreated WJ-MSCs. We also observed the dose-response effects of TGFβ on WJ-MSCs-derived exosomes. Therefore, exosomes derived from TGFβ-pretreated WJ-MSC may be critical in improving fibrosis and benefit liver fibrosis patients.