1. Background
Metabolic-associated fatty liver disease (MAFLD) is the leading cause of chronic liver disease worldwide. The burden of this disease is increasing due to the rising prevalence of metabolic syndrome (1). Similarly, the incidence of MAFLD in the Iranian population has increased in recent years (2). Considering the increasing burden of this disease, it is important to investigate its impact on the patients’ health-related quality of life (QoL). Besides, modification of factors that are related to QoL can improve the patients’ well-being.
A national survey in China showed that MAFLD impaired QoL (3). It could cause fatigue, depression, agitation, cognitive impairments, limited physical activity, and reduced well-being (4). Besides, obesity and diabetes mellitus (DM) decreased QoL, while achieving the appropriate body weight increased QoL in MAFLD patients (5, 6). Generally, QoL is adversely affected by lobular inflammation and advanced fibrosis, according to the histological examination of MAFLD (7-10). These findings suggest that QoL in MAFLD is correlated with disease severity and the associated metabolic disorders.
MAFLD is associated with cardiovascular disease (CVD) (11). Evidence suggests that the liver fat content, as a marker of visceral adiposity, is related to the development of CVD in MAFLD (12). CVD affects physical activity and may decrease QoL. Meanwhile, according to previous research, patients with at least one CVD risk factor, such as hypertension (HTN), DM, or high levels of cholesterol, are likely to describe their QoL as “less than good” (13). Overall, it seems reasonable to define CVD risk factors that impact QoL in MAFLD. Modification of these risk factors not only reduces the CVD morbidity and mortality, but also improves QoL in these patients.
So far, limited research has investigated the effect of single CVD risk factors on disability and QoL (14). There is also a paucity of literature on the relationship between multiple CVD risk factors and QoL in MAFLD. Notably, there is a lack of data addressing this phenomenon in the Iranian population.
2. Objectives
The current study aimed to evaluate the association between a panel of CVD risk factors [Framingham Risk Score (FRS)] and the main domains of QoL in a cohort of Iranian MAFLD patients.
3. Methods
3.1. Study Design
This cross-sectional, prospective cohort study was conducted on MAFLD patients, referred to the gastroenterology clinic of Sina Hospital, affiliated to Tehran University of Medical Sciences (Tehran, Iran), from September 2017 until September 2018.
3.2. Patient Enrolment Protocol
All patients with a persistent increase in aminotransferase levels above the normal range (40 U/L) and evidence of fatty liver on abdominal ultrasonography were enrolled in this study. The exclusion criteria were as follows: known chronic hepatitis (alcoholic fatty liver disease, viral disease, autoimmune disease, Wilson’s disease, and hemochromatosis); use of hepatotoxic medications; intravenous drug abuse; congestive heart failure; chronic kidney disease; chronic obstructive pulmonary disease; cirrhosis; and any known cancer, except skin cancer. MAFLD was diagnosed based on evidence of any fatty change on liver ultrasonography and ruling out other causes of a persistent increase in aminotransferase levels.
3.3. Sociodemographic Data Registry
The participants were interviewed, and their sociodemographic information, such as age, sex, marital status, education level, annual income, and smoking status, was registered. According to the poverty threshold defined by the research department of the Iranian Islamic Council, annual income was classified as low, low-intermediate, high-intermediate, and high (15). History of medical conditions, including HTN and DM, and history of receiving anti-hypertensive agents, oral hypoglycemic agents, or insulin were documented; this information was self-reported in the initial interview. The researcher examined the report validity by reviewing the participants’ medical records. Height (meter) and weight (kg) were measured, and the body mass index (BMI) was also calculated. BMI was categorized according to the World Health Organization (WHO) classification. A BMI of 18.5 to < 25 kg/m2 indicates a healthy range; a BMI of ≥ 25 to 30 kg/m2 represents the overweight range; a BMI of ≥ 30 to 35 kg/m2 represents the group of class 1 obesity; a BMI of ≥ 35 to 40 kg/m2 represents the group of class 2 obesity; and a BMI of ≥ 40 kg/m2 represents the group of class 3 obesity. Also, systolic blood pressure (SBP) at rest was recorded (cmHg) for each patient.
3.4. Laboratory Measurements
All laboratory measurements, including serum aspartate aminotransferase (AST, U/L), alanine aminotransferase (ALT, U/L), alkaline phosphatase (ALP, U/L), fasting blood sugar (FBS, mg/dL), triglyceride (TG, mg/dL), cholesterol (CHOL, mg/dL), low-density lipoprotein (LDL, mg/dL), and high-density lipoprotein (HDL, mg/dL), were performed based on the instructions of the manufacturers’ kits. These measurements were performed in the standard environment of the hospital medical laboratory. Laboratory values were defined based on the ELISA method, using a Roche 704 Automated Chemistry Analyzer. Also, Pars Azmun Reagent Kits (Tehran, Iran) were used for laboratory assessments, which were performed based on the manufacturer’s instructions described in our previous studies. To understand the measurement details and standard reporting units, readers are invited to study our previous research (16, 17).
3.5. Abdominal Ultrasonography Method
Transabdominal ultrasound was performed using a Hitachi EUB 405 apparatus, equipped with a 3.5 MHz convex probe to diagnose fatty liver. The radiologist compared the echogenicity of the right kidney (void of fat) with the right liver lobe in a sagittal view. The criteria for diagnosis and staging of fatty liver have been described in our previously published study (12).
3.6. CVD Risk Assessment
The sex-specific FRS was calculated for each participant, using a web-based FRS calculator (https://www.qxmd.com/calculate/calculator_252/framingham-risk-score-2008). This model uses age, smoking, diabetes, total cholesterol, HDL, HTN, and systolic blood pressure to estimate 10-year total coronary heart disease events (18). The results of calculations are expressed as percentage. The scores were classified as follows: < 10% as low-risk, 10 - 19% as intermediate risk, and ≥ 20% as high risk, according to the American Heart Association (AHA) CVD risk assessment guidelines (19, 20).
3.7. Health-Related QoL Evaluation
The QoL was evaluated using the Persian version of the WHOQOL-BREF questionnaire, validated in Iran (21). The WHOQOL-BREF is a tool, containing 26 items. Twenty-four items of the questionnaire distinguish physical health, psychological health, social relationships, and environment, and the last two items evaluate general health. The score for each domain and the overall score were recorded for each patient (22).
3.8. Ethical Considerations
The research goals were fully explained to patients who were willing to participate in this study, and a written informed consent form was obtained before enrollment. The Ethics Committee of Tehran University of Medical Sciences approved the protocol of this study (registration ID: IR.TUMS.DDRI.REC.1397.001).
3.9. Sample Size Calculation
At an alpha level of 0.05 (Zα = 1.96) and power of 80% (Zβ = 0.84), the biostatistician estimated the sample size based on the following formula:
The expected correlation coefficient (clinically significant) was set at a minimum level of 0.2. The sample size was estimated at 200.
3.10. Statistical Analysis Method
Demographic and clinical data are described as frequency (percentage) for categorical variables and as mean ± standard deviation (SD) for continuous variables. The normal distribution of variables was evaluated using Kolmogorov–Smirnov test, which indicated the non-normal distribution of all variables. Therefore, Mann–Whitney U test and Spearman's correlation test were used to analyze the associations between CVD risk factors and QoL in a univariate model. After adjusting for factors affecting QoL, a multiple linear regression analysis was performed to distinguish CVD risk factors, which were independently related to the QoL domains. The bootstrapping method was also applied by calculating the bias-corrected and accelerated (BCa) confidence intervals (CIs) with 1000 replications.
A two-step hierarchical multiple linear regression model was developed to determine the effect of each CVD risk factor on QoL while controlling for independent sociodemographic variables. In the first step, marital status, education level, income, geographical distribution, and BMI were entered in the model. In the second step, statistically significant variables (P < 0.05), including age, smoking, DM, HTN, and HDL, were entered into the model. Multicollinearity was assessed using the variance inflation factor (VIF) and tolerance for the variables. The tolerance values ranged from 0.108 to 0.959, and the VIF values ranged from 1.043 to 9.220; these findings revealed that multicollinearity was not a problem in the analyses. IBM SPSS Version 20 was used for data analysis. The significance level was set at P ≤ 0.05, and clinically significant correlations were set at ≥ 0.2. Also, regression coefficients with 95% CIs were reported for each regression model.
4. Results
4.1. Patient Enrollment Process
The eligibility of 254 patients, who were referred to our hospital for the evaluation of MAFLD during the study, was examined. Forty-one candidates did not meet the inclusion criteria, and seven candidates accepted the study protocol, but refused to sign the informed consent form. Of the remaining patients (n = 206), six did not complete the interviews or measurements; the participation rate was estimated at 97%.
4.2. Clinical and Sociodemographic Characteristics
This study was performed on 200 individuals (54% male; 46% female). The participants’ mean age was 51.64 ± 2.69 years (range: 40 - 65 years). Considering the geographical distribution, 26 (13%) patients resided in rural areas. The majority of the participants were married (71%, n = 142). In terms of education level, almost half of the participants had a master’s degree (49%, n = 98). Low income (11.5%, n = 23) and low-middle income (47.5%, n = 95) had the highest frequencies in the study population, respectively. The BMI was normal in 5 (5.5%) cases, overweight in 58 (29%) cases, and obese in 131 (94.5%) cases. The majority of the patients had DM (82%, n = 163) or HTN (77%, n = 154). The prevalence of smoking was 52 (26%). The participants’ mean systolic blood pressure was 14.19 ± 0.54 cmHg, with a range of 13 - 16 cmHg.
4.3. Laboratory Measurements, FRS Scores, and QoL Domain Scores
The mean serum cholesterol level was 182.25 ± 35.91 mg/dL (range: 107 - 387 mg/dL), and the mean serum HDL level was 34.55 ± 3.63 (range: 27 - 55 mg/dL). Based on the FRS, there were 20 (10%) individuals with a low risk of CVD development, 25 (12.5%) individuals with an intermediate risk, and 155 (77.5%) individuals with a high risk of CVD development. The mean FRS was 33.24% ± 15.91, with a range of 3.77% to 78.16% in the study population. The mean normalized QoL score of the participants was 43.55 ± 9.27, according to the WHOQOL-BREF questionnaire. The mean score of the physical domain was 44.79 ± 9.40; the mean score of the psychological domain was 40.40 ± 7.45; the mean score of the social relationship domain was 42.63 ± 14.62; and the mean score of the environmental domain was 45.17 ± 8.88. Table 1 presents the sociodemographic characteristics, clinical characteristics, QoL, and FRS scores in the study population.
| Characteristics | N = 200 | Percentage/Range |
|---|---|---|
| Age (y) | 51.64 ± 2.69 | 40 - 65 |
| Sex | ||
| Male | 108 | 54.00 |
| Female | 92 | 46.00 |
| BMI | ||
| Normal | 5 | 5.50 |
| Overweight | 58 | 29.00 |
| Obesity I | 86 | 43.00 |
| Obesity II | 32 | 16.00 |
| Obesity III | 13 | 6.50 |
| Geographical distribution | ||
| Rural | 26 | 13.00 |
| Urban | 174 | 87.00 |
| Marital status | ||
| Single | 29 | 14.50 |
| Married | 142 | 71.00 |
| Divorced | 23 | 11.50 |
| Widowed | 6 | 3.00 |
| Education level | ||
| Under high school diploma | 31 | 15.50 |
| High school diploma | 65 | 32.50 |
| Master’s degree | 98 | 49.00 |
| PhD | 6 | 3.00 |
| Income status | ||
| Low | 23 | 11.50 |
| Low-middle | 95 | 47.50 |
| High-middle | 65 | 32.50 |
| High | 17 | 8.50 |
| Smoking | ||
| Yes | 52 | 26.00 |
| No | 148 | 74.00 |
| DM | ||
| Yes | 163 | 82.00 |
| No | 37 | 18.00 |
| HTN | ||
| Yes | 154 | 77.00 |
| No | 46 | 23.00 |
| SBP (cmHg) | 14.19 ± 0.54 | 13 - 16 |
| Total cholesterol (mg/dL) | 182.25 ± 35.91 | 107 - 387 |
| HDL (mg/dL) | 34.55 ± 3.63 | 27 - 55 |
| FRS score | 33.24% ± 15.91 | 3.77 - 78.16 |
| Low risk (< 10%) | 20 | 10.00 |
| Intermediate risk (10 - 19%) | 25 | 12.50 |
| High risk (≥ 20%) | 155 | 77.50 |
| QoL score | 43.55 ± 9.27 | 20 - 55 |
| Physical domain | 44.79 ± 9.40 | 25 - 57 |
| Psychological domain | 40.40 ± 7.45 | 21 - 50 |
| Social relationships domain | 42.63 ± 14.62 | 0 - 58 |
| Environmental domain | 45.17 ± 8.88 | 22 - 56 |
Abbreviations: BMI, body mass index; cmHg, centimeter mercury; DM, diabetes mellitus; FRS, Framingham risk score; HDL, high-density lipoprotein; HTN, hypertension; mg/dL, milligram/deciliter; Qol; quality of life; SBP, systolic blood pressure.
a Values of quantitative variables are reported as mean ± SD.
b BMI is categorized according to the World Health Organization (WHO) classification. Healthy BMI range: 18.5 to < 25 kg/m2, overweight: ≥ 25 to 30 kg/m2; obese class I: ≥ 30 to 35 kg/m2, obese class II: ≥ 35 to 40 kg/m2, obese class III: ≥ 40 kg/m2.
4.4. Univariate Regression Analysis to Determine the Relationship Between QoL and CVD Risk Factors
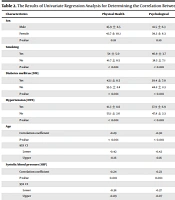
There was no significant difference in terms of QoL between men and women. However, the mean total QoL score and the mean score of each QoL domain were slightly higher in men than in women (P > 0.05). The univariate regression analysis showed a significant negative correlation between the overall QoL score and the FRS, age, smoking, DM, HTN, and SBP (P < 0.05). Moreover, a significant negative relationship was found between the mentioned CVD risk factors and all QoL domains in this analysis (P < 0.05). Meanwhile, the mean HDL level had a significant positive association with the overall QoL score and the scores of all QoL domains (P < 0.05). Table 2 presents the results of the univariate regression analysis for determining the relationship between QoL and CVD risk factors.
| Characteristics | Physical Health | Psychological | Social Relationships | Environment | Overall QoL |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 45.9 ± 8.5 | 41.5 ± 6.3 | 44.6 ± 12.8 | 46.3 ± 7.8 | 44.8 ± 8.1 |
| Female | 43.7 ± 10.1 | 39.3 ± 8.3 | 40.7 ± 16.1 | 44.0 ± 9.8 | 42.3 ± 10.2 |
| P-value | 0.18 | 0.10 | 0.14 | 0.16 | 0.15 |
| Smoking | |||||
| Yes | 54 ± 5.0 | 46.9 ± 3.7 | 55.5 ± 5.7 | 53.5 ± 4.1 | 52.3 ± 4.3 |
| No | 41.7 ± 8.5 | 38.2 ± 7.1 | 38.3 ± 14.4 | 42.4 ± 8.3 | 40.6 ± 8.6 |
| P-value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| DM | |||||
| Yes | 42.1 ± 8.5 | 38.4 ± 7.0 | 39.9 ± 14.0 | 42.7 ± 8.2 | 41.0 ± 8.6 |
| No | 55.5 ± 2.8 | 48.2 ± 2.3 | 57.7 ± 2.2 | 54.9 ± 2.1 | 53.8 ± 2.2 |
| P-value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| HTN | |||||
| Yes | 41.3 ± 8.6 | 37.9 ± 6.9 | 37.9 ± 13.8 | 42.1 ± 8.1 | 40.3 ± 8.4 |
| No | 55.1 ± 2.6 | 47.8 ± 2.3 | 56.8 ± 3.2 | 54.5 ± 2.1 | 53.3 ± 2.2 |
| P-value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Age | |||||
| Correlation coefficient | -0.29 | -0.30 | -0.28 | -0.30 | -0.29 |
| P-value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| 95% CI | |||||
| Lower | -0.42 | -0.42 | -0.41 | -0.42 | -0.42 |
| Upper | -0.16 | -0.16 | -0.13 | -0.16 | -0.15 |
| SBP | |||||
| Correlation coefficient | -0.24 | -0.23 | -0.24 | -0.26 | -0.25 |
| P-value | 0.001 | 0.001 | 0.001 | < 0.001 | < 0.001 |
| 95% CI | |||||
| Lower | -0.38 | -0.37 | -0.38 | -0.39 | -0.39 |
| Upper | -0.09 | -0.07 | -0.09 | -0.10 | -0.10 |
| Total cholesterol | |||||
| Correlation coefficient | 0.03 | 0.03 | 0.01 | 0.02 | 0.02 |
| P-value | 0.67 | 0.71 | 0.89 | 0.75 | 0.74 |
| 95% CI | |||||
| Lower | -0.11 | -0.11 | -0.14 | -0.13 | -0.12 |
| Upper | 0.18 | 0.18 | 0.17 | 0.19 | 0.18 |
| HDL | |||||
| Correlation coefficient | 0.18 | 0.18 | 0.19 | 0.19 | 0.19 |
| P-value | 0.013 | 0.009 | 0.006 | 0.008 | 0.008 |
| 95% CI | |||||
| Lower | 0.01 | 0.03 | 0.04 | 0.02 | 0.02 |
| Upper | 0.31 | 0.32 | 0.34 | 0.31 | 0.32 |
| FRS | |||||
| Correlation coefficient | -0.49 | -0.45 | -0.48 | -0.50 | -0.49 |
| P-value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| 95% CI | |||||
| Lower | -0.60 | -0.57 | -0.60 | -0.61 | -0.61 |
| Upper | -0.34 | -0.32 | -0.34 | -0.36 | -0.35 |
Abbreviations: DM, diabetes mellitus; FRS, Framingham risk score; HDL, high-density lipoprotein; HTN, hypertension; Qol; quality of life; SBP, systolic blood pressure.
4.5. Hierarchical Multiple Linear Regression Model to Determine the Effect of Each CVD Risk Factor on QoL
Table 3 presents the results of adjusted regression models for defining the association of QoL scores with the CVD risk (model 1) and FRS (model 2). There was a significant negative association between the overall QoL score and age (B = -0.21; 95% CI: -0.41, -0.02; P < 0.05), smoking (B = -2.44; 95% CI: -3.73, -1.11; P < 0.05), DM (B = -2.44, 95% CI: -3.87, -1.28; P < 0.05), HTN (B = -5.51; 95% CI: -7.18, -3.68; P < 0.05), and FRS (B = -0.04; 95% CI: -0.08, -0.02; P < 0.05). HTN was the main predictor of the total QoL score. Smoking, DM, and HTN were the key determinants of all domains of QoL. Age was inversely associated with the phycological domain of QoL (B = -0.18; 95% CI: -0.35, -0.01; P < 0.05), social relationships domain (B = -0.36; 95% CI: -0.69, -0.03; P < 0.05), and environmental domain (B = -0.24; 95% CI: -0.47, -0.05; P < 0.05). A higher FRS score was inversely associated with the physical domain of QoL (B = -0.05; 95% CI: -0.09, -0.01; P < 0.05) and the environment domain of QoL (B = -0.04; 95% CI: -0.09, -0.01; P < 0.05).
| Variables | Physical Health | Psychological Health | Social Relationships | Environment | Overall |
|---|---|---|---|---|---|
| Model 1 (QoL and CVD Risk) | |||||
| Age | |||||
| Regression coefficient | -0.13 | -0.18 | -0.36 | -0.24 | -0.21 |
| 95% CI | (-0.27, 0.01) | (-0.35, -0.01) | (-0.69, -0.03) | (-0.47, -0.05) | (-0.41, -0.02) |
| P-value | 0.07 | 0.04 | 0.03 | 0.02 | 0.02 |
| Smoking | |||||
| Regression coefficient | -2.64 | -2.3 | -2.74 | -2.26 | -2.44 |
| 95% CI | (-4.87, -0.54) | (-3.41, -1.07) | (-5.32, -0.81) | (-3.36, -1.09) | (-3.73, -1.11) |
| P-value | 0.005 | 0.001 | 0.01 | 0.002 | 0.001 |
| DM | |||||
| Regression coefficient | -1.62 | -2.41 | -4.7 | -2.35 | -2.44 |
| 95% CI | (-3.05, -0.48) | (-3.78, -1.28) | (-7.66, -1.64) | (-4.11, -1.03) | (-3.87, -1.28) |
| P-value | 0.009 | 0.001 | 0.002 | 0.002 | 0.001 |
| HTN | |||||
| Regression coefficient | -6.78 | -3.71 | -5.66 | -5.67 | -5.51 |
| 95% CI | (-9.38, -4.61) | (-5.12, -2.29) | (-9.46, -2.29) | (-7.41, -3.82) | (-7.18, -3.68) |
| P-value | 0.001 | 0.001 | 0.003 | 0.001 | 0.001 |
| HDL | |||||
| Regression coefficient | 0.01 | 0.01 | 0.01 | 0.04 | 0.01 |
| 95% CI | (0.01, 0.03) | (-0.01, 0.02) | (-0.01, 0.04) | (0.00, 0.03) | (0.00, 0.02) |
| P-value | 0.29 | 0.26 | 0.23 | 0.08 | 0.1 |
| Model 2 (QoL and FRS) | |||||
| FRS | |||||
| Regression coefficient | -0.05 | -0.02 | -0.05 | -0.04 | -0.04 |
| 95% CI | (-0.09, -0.01) | (-0.05, 0.02) | (-0.11, 0.02) | (-0.09, -0.01) | (-0.08, -0.02) |
| P-value | < 0.01 | 0.42 | 0.19 | 0.04 | 0.04 |
Abbreviations: CVD, cardiovascular Disease; DM, diabetes mellitus; FRS, Framingham risk score; HDL, high-density lipoprotein; HTN, hypertension; Qol; quality of life.
5. Discussion
The present study evaluated the association between QoL and CVD risk factors in a cohort of Iranian MAFLD patients. The CVD risk factors were prevalent in the study population. The FRS score was inversely associated with QoL. The results of the univariate regression analysis revealed significant correlations between six out of eight factors present in the FRS equation and the score of QoL. After controlling for confounding factors affecting QoL, the results of the hierarchical multiple linear regression model showed that four of these factors had significant effects on QoL. HTN was the best predictor of QoL in all domains, as well as the overall QoL. DM and smoking were the second and third best predictors of QoL in this study, respectively. The strength of the current study was the simultaneous evaluation of a panel of CVD risk factors while adjusting for the main factors influencing QOL.
The methodological differences in the sociodemographic characteristics of the participants in earlier studies might have produced conflicting results regarding the association between CVD and QoL. Using the WHOQOL-BREF questionnaire, Blay et al. found that age was not associated with QoL in any of the four domains (23). On the other hand, Garcia-Campayo et al. used the short-form health survey (SF-36) and concluded that age significantly affected the mental aspect of QoL; however, it was not possible to determine the association between the physical aspect of QoL and age (24). In the present study, age played a significant role in predicting the environmental QoL score, as well as the total score of QoL in patients with MAFLD. Although this study did not include a wide age range, the results showed that attention must be paid to the environmental factors in the elderly as their age advances. Meanwhile, sex was not significantly associated with the QoL scores in our survey, which is consistent with the results of previous research (23, 25).
Among clinical characteristics, HTN was the most important factor in determining QoL in the present study. Similarly, a systematic review by Trevisol et al. showed that both physical and mental health domains of QoL were inversely related to HTN (26). This meta-analysis compared QoL between hypertensive and normotensive individuals by extracting the data of 20 eligible studies. Lower scores of the physical and mental domains were reported based on FS-36 in the hypertensive group. Moreover, Uchmanowicz et al. found that lower QoL was associated with reduced adherence to therapeutic recommendations (27). Their study on 186 hypertensive elderly patients showed that QoL scores dramatically decreased in patients with low compliance regarding “appointment keeping” and “medication taking”, which in turn exacerbated the disease over time and led to an even lower QoL.
The majority of patients with HTN either had a high risk of CVD or had already developed CVD. The results of a trial on 207 hypertensive individuals revealed that HTN management not only reduced the CVD risk, but also improved the QoL (28). Similar to our findings, Oza et al. found that SBP was negatively associated with QoL (29). This study included 269 hypertensive patients and assessed QoL using the WHOQOL-BREF and MINICHAL scales. On the other hand, Katsi et al. described that stage and awareness of hypertension did not affect physical and mental health (30). This survey used SF-36 for the evaluation of QoL and included 189 hypertensive patients. The controversial results of the mentioned research could be attributed to differences in the study population regarding the socioeconomic factors and the type of questionnaire used for QoL evaluation.
Similarly, DM was another determinant of QoL among the participants, which influenced all domains of QoL and the overall score of QoL. Consistent with the results of our survey, Trikkalinou et al. found that DM affected major components of QoL. They proposed that DM comorbidities and psychological burden caused limitations in their communication with friends and social ties (31). It is noteworthy that in the present study, DM affected social relationships more than any other domain. Additionally, Goldenberg et al., in a meta-analysis evaluating 54 studies, reported that smokers had an impaired QoL in their lifetime. The extent of this negative association was related to the number of cigarettes smoked per day (32). This finding is consistent with our results, which showed that smoking had negative effect on QoL.
To the best of our knowledge, there is no information in the literature regarding the relationship between dyslipidemia and QoL in MAFLD patients. However, there are controversies considering the association of QoL with dyslipidemia in non-MAFLD cohorts (33, 34). A survey by Zhang et al. on 756 post-myocardial infarction patients showed that lipid control could improve QoL; they measured the EQ-5D score for the assessment of QoL (33). On the contrary, Souto et al. showed that familial hypercholesterolemia (FH) was not associated with QoL (34). This study included individuals with a high risk of FH, undergoing genetic screening. The QoL was measured using SF-12 before molecular tests. The mean QoL scores were not significantly different between affected and non-affected cases of FH. Neither the presence of mutations, nor pharmacological treatments were related to QoL; however, a history of CVD was negatively related to QoL. Meanwhile, no significant association was found between QoL and HDL or total cholesterol in a sample of MAFLD patients with a moderate CVD risk. The discrepancy between the results of previous research might be related to differences in the study population characteristics regarding the CVD risk and determinants of QoL.
There are some limitations to the present study. First, this was a cross-sectional study; therefore, it was not possible to infer any causality or directionality. Second, due to the use of convenience sampling, the results may not be generalizable to all MAFLD patients. Third, some gathered data was self-reported in this study, which might result in recall (reporting) bias. Finally, some clinical features (anxiety state or depressive mood) that might influence QoL were not assessed in the current study. To overcome these limitations, multicenter cohort studies with a larger sample size are recommended while considering the participants’ moods and emotions.
5.1. Conclusion
According to the current results, HTN, DM, and smoking were the main predictors of QoL in a cohort of Iranian MAFLD patients. Based on the results, control of the mentioned risk factors in these patients would not only improve the QoL, but also reduce the CVD mortality and morbidity.