1. Introduction
Both chronic hepatitis B (HBV) and chronic hepatitis C (HCV) infections are major public health issues globally (1). The primary concern is HBV and HCV co-infection that results in a more severe liver disease progression compared to mono-infections (2-4), with an increased risk of hepatocellular cancer (HCC) (5-7) and of fulminant hepatitis (8). New findings confirmed that HBV/HCV coinfected patients might experience more rapid progression of severe liver disease compared to those with hepatitis B alone although similar data for Asian and African patients with the highest prevalence of HBV infection worldwide are still missing (9). Furthermore, co-infection did not worsen HCV liver disease progression in the same study (9). Nevertheless, treatment should be prioritized for HCV/HBV dually infected patients to avoid severe liver disease progression (10). Recently published recommendations from both the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) suggest that the same therapeutic criteria should be applied to patients who are co-infected based on the viral dominance after hepatitis B and C viral interactions as applied to patients with either HBV or HCV monoinfection (10, 11). The introduction of new direct-acting drugs (DAAs) has brought new therapeutic options in treating HCV although they need to be evaluated in co-infected patients with hepatitis B and C viruses. Nevertheless, it seems reasonable as a first-line therapy based on existing data for DAAs therapies in HCV mono-infected patients. A shift to a nucleos(t)ide analogue with high potency and high genetic barrier for patients with HBV DNA persistence should always be considered. Rarely, both HBV and HCV treatments could be implemented in case of reactivation of hepatitis B virus during anti-HCV treatment or after treatment or synchronously active HBC/HCV infection (10). However, the risk of HBV reactivation during DAAs-based anti-HCV treatment in HBV/HCV co-infection is unpredictable and needs to be evaluated. Here, we report the case of a 47-year-old female patient with HBV/HCV coinfection who experienced HBV reactivation during treatment with daclatasvir (DCV) and sofosbuvir (SOF).
2. Case Presentation
A 47-year-old female patient with HBV/HCV coinfection, known since 1993 of Limassol General Hospital, presented to our outpatient gastroenterology clinic for treatment. There was no history of previous treatment. She had HCV Genotype 3 with serum levels of alanine aminotransferase (ALT) of 112 U/L, γ-glutamyltransferase 53 U/L, bilirubin 1.1 mg/dL, HBsAg 1.1 log IU/L anti-HBc total 2.9 logU/mL, anti-HBc IgM negative, anti-HBe positive, serum HCV-RNA 4.8 logIU/mL, and serum HBV HBV-DNA of 1.1 logIU/mL. Liver ultrasound was negative for cirrhosis findings. Fibroscan revealed liver stiffness measurement 11 KPa.
HCV treatment with DCV (400 mg) and SOF (60 mg) daily for 12 weeks started because of moderate liver fibrosis (liver stiffness measurement: 11 KPa) and HCV dominance. The low serum HBV DNA levels (< 2000 IU/mL) and anti-HBc IgM negative serology suggested that she should be classified as a patient with HBeAg-negative chronic HBV infection, previously termed ‘inactive carrier’ (HBsAg positive (usually less than 1000 IU/mL), HBeAg (-), anti-HBe (+), very low or undetectable serum HBV DNA levels (< 2.000 IU/mL, occasionally < 20.000 IU/mL), and normal serum aminotransferases (12). Thus, HBV treatment was not thought to be necessary for her according to a recent publish by EASL clinical practice guidelines on the management of hepatitis B virus infection (12).
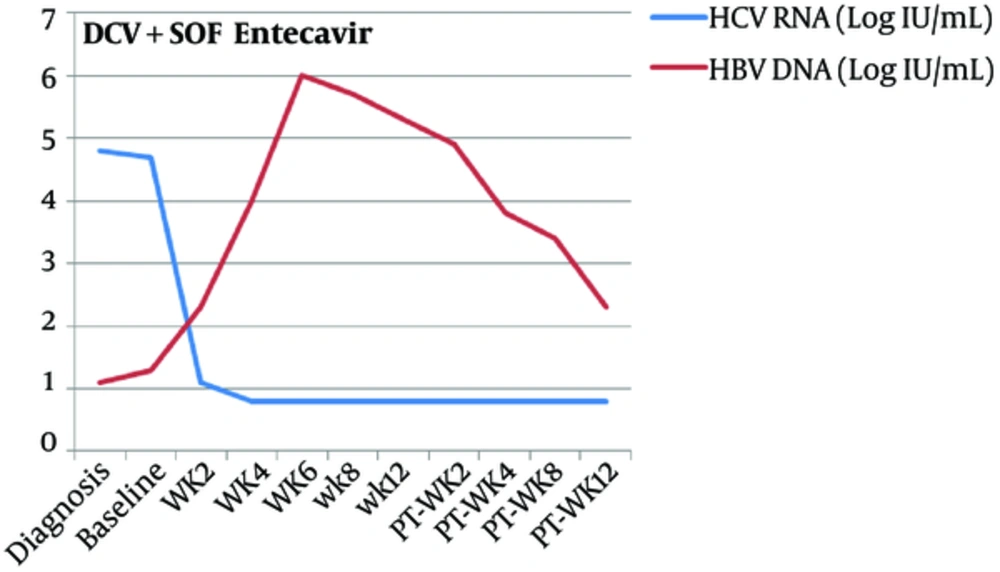
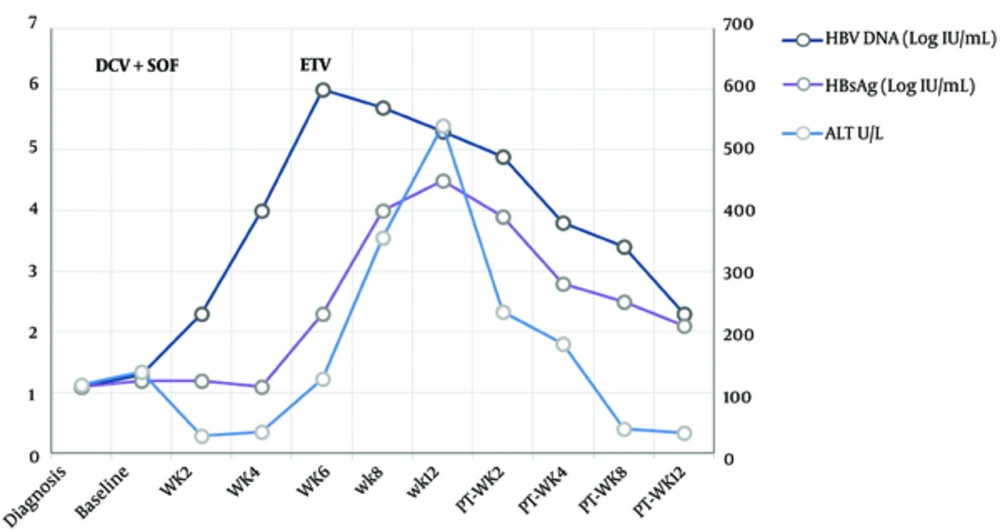
During HCV treatment, close monitoring of serum ALT showed a gradual increase that reached 123 U/L on week 6 although HCV RNA levels were undetectable. Serum HBV DNA was increasing to 6.0 logIU/mL at week 6. Anti-HBc IgM became positive at week 4. Total Bilirubin was always less than 3 mg/dL. Entecavir (0.5 mg daily) was added to the combined regimen with improvement in ALT levels.
| Variables | Value |
|---|---|
| WBCs | 6.7 × 109/L |
| Hb | 12.4 g/dL |
| PLT | 16.7 × 109/L |
| PT/INR | 91/1.1 s |
| Albumin | 4.1 g/dL |
| Total bilirubin | 1.1 mg/dL |
| AST/ALT | 98/112 U/L |
| gGT | 53 U/L |
| HBsAg | + |
| HBeAg | - |
| Anti-HBe | + |
| Anti-HBc total | + |
| Anti-HBc IgM | - |
| Anti-HCV | + |
| HCV genotype | 3 |
| HCV-RNA levels | 4.8 logIU/mL |
3. Discussion
In real life, the alternating replicative patterns of both viruses in coinfected HBV/HCV patients have a clinical impact. This is often because one of the viruses is replicating at a much faster rate, inhibiting the replication of the other. Indeed, in some patients with HBV/HCV dual infection after eradication of the dominant virus such as clearance of serum HCV RNA with pegylated interferon-α (peg-IFN-α) and ribavirin (RBV), the other virus then may become active (HBV reactivation) (13, 14). Furthermore, reactivation of hepatitis B during the recent use of DAAs in the treatment of hepatitis C in dual HBV/HCV infections seems to be a challenge.
Indeed, few interesting cases of HBV reactivation with the DAAs for HCV therapy have been recently reported. In the study of Takayama et al. (15), a dually infected patient initially received treatment with asunaprevir and daclatasvir for the HCV dominance, resulting in HCV early virological response; but the increase of serum HBV DNA levels required the use of a nucleoside analogue such as entecavir. We report a similar recent case of HBV reactivation of a patient coinfected with hepatitis B and C genotype 3 during treatment with Daclatasvir and Sofosbuvir as shown in Figures 1 and 2.
At baseline, the patient was positive for HBsAg and anti-HCV. Serum HCV RNA levels were 4.7 logIU/mL and low HBV DNA levels were 1.3 logIU/mL. After treatment with the combined regimens, serum HCV RNA levels declined. However, when HCV RNA became undetectable, HBV DNA began to rise and entecavir was added (week 6).
Another case also has shown early HBV reactivation during HCV treatment with the DAAs ledipasvir and sofosbuvir. The patient with past HBV infection had chronic hepatitis C infection (HCV genotype 4) and HIV coinfection (16).
Finally, similar three other cases of HBV reactivation were reported during HCV treatment with DAAs (sofosbuvir/simeprevir) in patients with HBV and HCV genotype 1 coinfections (17, 18). These three patients had a history of past treatment of their HCV infection with peginterferon/ribavirin without virological response. One patient was an inactive HBV carrier (serum HBV DNA levels < 2000 IU/mL or undetectable and normal serum aminotransferases), one had occult hepatitis B (HBsAg (-) and very low serum HBV DNA levels), and the last one had a past infection (HBsAg (-), anti-HBc (+), anti-HBs (±), and undetectable serum HBV DNA levels). During anti- HCV treatment, serum HBV DNA levels increased and subsequently a nucleos(t)ide analogue (tenofovir, emtricitabine or entecavir) was added. However, one patient had liver transplantation because of fulminant hepatic failure despite the addition of nucleos(t)ide analoque.
There has been also one report of HBV reactivation with the triple therapy of peginterferon-α, ribavirin, and simeprevir in a patient with HBV/HCV coinfection. Entecavir was administered in addition to triple anti-HCV treatment and serum HBV DNA levels became undetectable (19).
Overall, for the period from November 2013 to October 2016, the US food and drug administration (FDA) reported 29 cases of HBV reactivation in patients with dual HCV/HBV infection who received DAAs regimens as anti-HCV therapy (20). As a result, FDA now issued a warning about the risk of HBV reactivation in those patients who are going to be treated with DAAs.
Of those 29 patients, at baseline, 9 had detectable serum HBV DNA levels, 7 were HBsAg-positive and had undetectable serum HBV DNA levels, and 3 were HBsAg-negative and had undetectable HBV-DNA levels. For the remaining 10 patients, data were not available or uninterpretable. Two deaths and 1 case of liver transplantation were reported.
Although it is now clear that there is a potential risk for HBV reactivation associated with DAAs therapy, the risk is unpredictable. One recent study reported that out of 103 patients with resolved HBV infection treated with DAAs, none experienced reactivation (21). Another study reported out of 327 patients who received DAAs as antiviral therapy, 124 were anti-HBc positive while none of them showed HBV reactivation (22).
In conclusion, the current EASL and ASSLD HCV treatment guidelines suggest how to monitor these coinfected patients on DAAs with an unpredictable risk of HBV reactivation although the new regimens need to be evaluated in HBV/HCV-coinfected patients (10, 11). Concurrent HBV nucleoside/nucleotide analogue therapy seems to be indicated because of the unpredictable risk of HBV reactivation during HCV treatment with DAAs, especially in cases with high serum HBV DNA levels before the initiation of DAAs-based HCV treatment (10, 11). In resolved HBV infection, serum aminotransferase levels should be monitored and upon an increase in ALT levels on treatment, a test should be implemented for HBs antigen and/or serum HBV DNA levels. Close monitoring of HBV infection is necessary.