1. Introduction
The morbidity and mortality of coronavirus disease 2019 (COVID-19) infection increased in patients with chronic liver disease (CLD), and especially those with cirrhosis. The American Association for the Studies of Liver Diseases (AASLD) and the European Association of the Study of the Liver (EASL) recommended that COVID-19 vaccination should be prioritized in patients with CLD (1, 2). However, there are still uncertainties about the safety of COVID-19 vaccination in this group of patients. There were no reported cases of HBV flare after COVID-19 vaccination in the literature.
2. Case Presentation
2.1. Patient 1
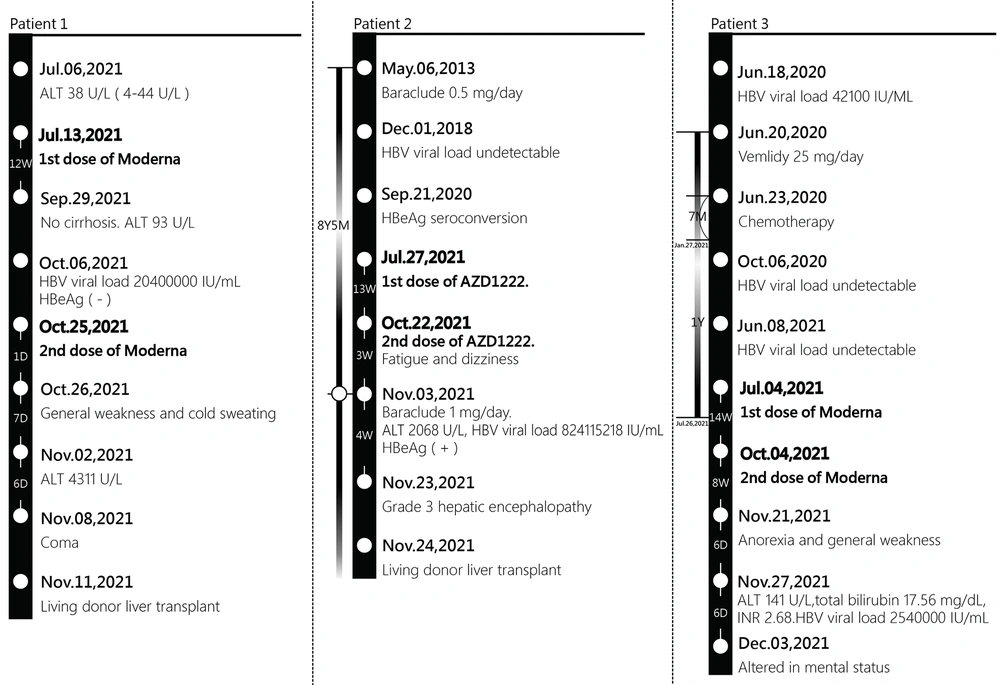
A 65-year-old male presented to emergency department on November 2nd, 2021, with 1-week history of general weakness and cold sweating the day after receiving second dose of mRNA-1273 (Moderna) on October 25th, 2021. The patient had medical history of HBV infection and hypertension. Reverse transcription real-time polymerase chain reaction (RT-PCR) test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was negative. On examination, his skin was jaundiced with icteric sclera. Serum aspartate aminotransferase (AST) showed 3774 U/L, alanine aminotransferase (ALT) 4311 U/L, total bilirubin level 8.61 mg/dL, direct bilirubin level 5.26 mg/dL, and international normalized ratio (INR) 4.58. Tracing his medical history, he received first dose of mRNA-1273 (Moderna) on July 13th, 2021. There were no discomforts related to it. The patient had been followed up in a community hospital for inactive HBV infection for more than ten years. The serum ALT level was within normal limits during follow-up. ALT was 38 U/L (4 to 44 in normal range) on July 6th, 2021, which increased to 93 U/L without cirrhosis by abdominal sonography on September 29th, 2021. HBV viral load was 20400000 IU/mL with hepatitis B e antigen (HBeAg) negative on October 6th, 2021. Entecavir (Baraclude) 1 mg/day was prescribed on November 2nd, 2021. The next day, he developed flapping tremor, and his mental status altered. On November 4th, 2021, INR increased to 6.39, and serum total bilirubin level increased to 12.56 mg/dL. Acute-on-chronic liver failure (ACLF) due to HBV reactivation was impressed. On November 8th, 2021, the patient was comatose, and he underwent urgent living donor liver transplantation on November 11th, 2021.
2.2. Patient 2
A 52-year-old male presented to emergency department on November 3rd, 2021, with persistent fatigue and dizziness after second dose of ChAdOx1 nCoV-19 (AZD1222) on October 22nd, 2021. On examination, his skin was jaundiced with icteric sclera. He had negative RT-PCR test for SARS-CoV-2. The serum ALT was 2068 U/L, AST was 1594 U/L, and total bilirubin was 6.83 mg/dL. HBV viral load was 824115218 IU/mL with HBeAg positive. Tracing his medical history, he received first dose of ChAdOx1 nCoV-19 (AZD1222) on July 27th, 2021. There were no discomforts related to it. The patient was on 0.5 mg/day Entecavir (Baraclude) since May 6th, 2013 for chronic hepatitis B. HBV viral load was undetectable since December 1st, 2018, and he was HBeAg negative (seroconversion) on September 21st, 2020. Furthermore, serum AST and ALT were within normal limits during follow-up visits. We increased the dose of Entecavir (Baraclude) to 1 mg/day for HBV flare. Sonography and computed tomography (CT) scan of liver did not reveal cirrhosis, and the amount of ascites was small. There was no drug resistance revealed by DNA sequencing analysis. The total bilirubin level and INR increased to 32.67 mg/dL and 3.1, respectively, refractory to medical treatment, and he developed grade 3 hepatic encephalopathy on November 23rd, 2021. The patient underwent urgent living donor liver transplant on November 24th, 2021.
2.3. Patient 3
A 73-year-old female presented with 1-week history of anorexia and general weakness on November 27th, 2021. On examination, the patient was jaundiced with icteric sclera and had mild confusion. The serum ALT was 141 U/L, AST was 297 U/L, total bilirubin level was 17.56 mg/dL, direct bilirubin level was 14.10 mg/dL, and INR 2.68. HBV viral load was 2540000 IU/mL. She had negative RT-PCR test for SARS-Cov-2. Tracing her medical history, the patient was diagnosed with stage IV mantle cell lymphoma (MCL) on June 13th, 2020, and she was under chemotherapy with bendamustine plus rituximab from June 23rd, 2020, to January 27th, 2021. She took prophylactic Tenofovir alafenamide (vemlidy) 25 mg/day from June 20th, 2020, to July 26th, 2021. The HBV viral load before treatment was 42100 IU/ML and soon turned undetectable. She received Tenofovir alafenamide (vemlidy) for six more months after completing chemotherapy with bendamustine plus rituximab treatment. The serum AST and ALT were within normal limits during follow-up visits. There was complete response to treatment and no recurrent evidence of MCL on presentation. She had other medical history, including hypertension and type 2 diabetes mellitus. The patient received second dose of mRNA-1273 (Moderna) on October 4th, 2021, and first dose on July 4th, 2021. Tenofovir alafenamide (vemlidy) was discontinued three weeks after first dose of mRNA-1273 (Moderna). No specific discomforts were reported after the first dose of mRNA-1273 (Moderna). On presentation, ACLF due to HBV reactivation was impressed, and we initiated Entecavir (Baraclude) 1 mg/day. The mental status altered on December 3rd, 2021, and total bilirubin and INR increased to 21.05 mg/dL and 2.43, respectively. She was not candidate for liver transplantation due to old age.
The SARS-Cov-2 antibody was tested in our patients by Elecsys Anti-SARS-CoV-2 S immunoassay. The results of antibody for Patient 1 and Patient 2 were 1689.00 U/mL and 765.00 U/mL, respectively, which was interpreted as positive. Patient 3 refused the test due to personal reason.
Among our three patients, virus markers were negative for hepatitis A, C, and D. Anti-mitochondrial antibodies (AMA) and anti-smooth muscle antibodies (ASMA) were negative in all patients. Antinuclear antibody (ANA) was negative in Patient 1 and Patient 3 and positive (1:80, speckle and homogenous) in Patient 2. The liver pathology report of Patient 1 showed submassive necrosis, and Patient 2 showed submassive necrosis with cirrhosis. There was no evidence of autoimmune hepatitis. Figure 1 summarizes the study patients.
Timeline of hepatitis B virus status relative to COVID-19 vaccination. Moderna: mRNA-1273 vaccine, AZD1222: ChAdOx1 nCoV-19, ALT: alanine aminotransferase, HBV: hepatitis B virus, HBeAg: hepatitis B e antigen, Baraclude: Entecavir, vemlidy: Tenofovir alafenamide, INR: international normalized ratio.
3. Discussion
In patients with COVID-19, preliminary data suggested that CLD of any etiology was associated with worse outcomes (3, 4). The adverse effects related to COVID-19 vaccination are mostly mild and limited to injection site pain, fatigue, headache, myalgia, chills, and fever (5, 6). However, there were insufficient data supporting the safety of vaccination. Rare patients of autoimmune hepatitis or autoimmune-like hepatitis were reported within days to weeks after receiving mRNA-based COVID-19 vaccines (7, 8).
The safety of COVID-19 inactivated vaccine in patients with chronic HBV infection were also studied. The adverse effects were similar between patients with chronic HBV infection and healthy population (9). No serious side effects were observed even in patients with compensated liver cirrhosis and the immune response was better in patients receiving antiviral therapy for chronic HBV infection (10).
According to the AASLD 2018 guideline, patients with HBV infection receiving chemotherapy or immunosuppressive therapy should be administered antiviral therapy before and at least six to twelve months (at least twelve months for anti-CD20 therapy) after completing chemotherapy or immunosuppression (11). Tenofovir alafenamide (vemlidy) was discontinued six months after completion of chemotherapy with bendamustine plus rituximab treatment with undetectable HBV viral load in Patient 3. We discontinued Tenofovir alafenamide (vemlidy) after six months but within twelve months after completing rituximab, which could be criticized. However, the HBV viral load was undetectable at that time, and the risk of reactivation of HBV infection should therefore be low.
COVID-19-related HBV reactivation has been reported in some previous studies (12-14). SARS-CoV-2-induced extreme inflammatory response is one of the explanations, and the immunosuppressive treatment for COVID-19 may also contribute to it. Furthermore, co-infection of SARS-CoV-2 and HBV poses risk of greater liver injury. The potential factors include direct liver injury by SARS-CoV-2, hepatotoxic antiviral drugs during COVID-19 therapy, and immune-mediated inflammatory response post SARS-CoV-2 co-infection (14). Hepatitis C virus (HCV) reactivation following mRNA-based Pfizer-BioNTech COVID-19 vaccine has also been reported (15). Reactivation of HBV after mRNA-based or chimpanzee adenovirus-vectored COVID-19 vaccines was not previously reported. To the best of our knowledge, this is the first reported episode of ACLF due to HBV reactivation after COVID-19 vaccination. The mechanism behind this issue is unclear. One possible explanation is that COVID-19 vaccination influences the normal immune response to HBV and causes subsequent reactivation.
New virus variants keep coming out and extra doses of COVID-19 vaccine is considered in this setting. According to our findings, prophylactic antiviral therapy prior to vaccination for individuals with HBV infection should be considered.
In summary, we presented three patients with ACLF suspected due to HBV reactivation after COVID-19 vaccination. Two of them received urgent living donor liver transplantation, and one was not qualified for her old age (73 years old). The cause-and-effect relationship between COVID-19 vaccination and HBV infection flare was not established, and the mechanism remained unknown. In this regard, further studies are needed. Moreover, during the COVID-19 pandemic, prophylactic antiviral therapy for individuals with HBV infection prior to vaccination deserves attention.