1. Background
Acute liver failure (ALF) is caused by massive hepatocyte death and accompanied by coagulation disorder and encephalopathy. It often leads to multiple organ failure and, subsequently death. Thus, the underlying mechanism of ALF in affecting other organs remains unclear. The elevation of pancreatic enzyme was often observed in acute liver injury (ALI) patients. Therefore, we focused on the elevation of pancreatic enzymes in ALI patients. It is reported that acute pancreatitis is often accompanied by ALI (1-3). This is mainly reported as a complication of viral hepatitis (4, 5). However, nonviral ALI has also been reported to be accompanied by acute pancreatitis (2). At present, the association between the development of pancreatic disorder and ALI remains unclear. To clarify this association, we analyzed patients with ALI and elevated pancreatic enzymes in this study.
2. Objectives
This study aimed to reveal the association between the elevation of pancreatic enzymes and acute liver injury.
3. Methods
3.1. Patients
This study is a single-center retrospective study to analyze patients with ALI treated at Kyushu University Hospital between 2012 and 2017. Blood samples were used to assess the liver function, coagulation profile, immunological parameters, and viral markers such as hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis E virus (HEV), cytomegalovirus, herpes simplex virus (types 1 and 2), and Epstein-Barr virus. At discharge, autoimmune hepatitis (AIH) diagnoses were confirmed according to the revised criteria of the International Autoimmune Hepatitis Group. We excluded the extra-hepatic cholestasis by CT imaging. Patients with malignant tumors and liver cirrhosis who had been previously diagnosed by blood tests or imaging were excluded from this study. ALF was diagnosed by the criteria established by the Intractable Hepato-Biliary Diseases Study Group in Japan (6). Patients who had previously normal liver functions and progressed to severe liver damage with prothrombin activity percentages (PT%) of 40% or less of the standardized values or international normalized ratios (INRs) of 1.5 or more within eight weeks of the onset of symptoms were diagnosed with ALF. Moreover, we further classified these patients into ALF with or without hepatic coma. In the former, hepatic encephalopathy grade II or more severe hepatic coma developed within eight weeks. ALF with hepatic coma was subclassified into two groups by the timing of development of grade II or more severe hepatic encephalopathy: The patients with severe hepatic encephalopathy developing within ten days after the onset of disease symptoms were classified as acute ALF, and the patients with severe hepatic encephalopathy developing between 11 and 56 days of the onset of disease symptoms were classified as subacute ALF. Patients who had prothrombin time values of less than 40% of the standardized values or INRs of 1.5 or more and grade II or more severe hepatic coma between eight and 24 weeks of the onset of disease symptoms were diagnosed as having late-onset hepatic failure (LOHF) (6). The patients with obvious liver atrophy and hepatic coma were immediately prepared for liver transplantation (LT). We treated enrolled patients with comprehensive supportive care such as plasma exchange, continuous hemodiafiltration, and/or anticoagulant therapy, including recombinant thrombomodulin and antithrombin III as necessary. Using the elevation of serum amylase or lipase above 1.5 times the upper limit of normal as a cut-off value, patients were divided into two groups: Elevation group and no elevation group.
3.2. Measurement of Cytokines
Serum levels of tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), and interleukin-6 (IL-6) were measured using the Human TNF alpha ELISA Kit (Abcam), Human IFN gamma ELISA Kit (Abcam), and Human IL-6 ELISA Kit (Abcam), respectively.
3.3. Statistical Analysis
Data were analyzed with JMP software package (version 15.1.0; SAS Institute, Inc., Cary, NC, USA). Continuous variables are expressed as mean values and standard deviations or median and interquartile ranges. Unpaired student t-test, Fisher’s exact test, Wilcoxon signed-rank test, or χ2 test were used to assess significant differences between groups. A P-value of < 0.05 was considered statistically significant.
4. Results
4.1. Clinical Characteristics of ALI
In this study, 163 patients with ALI were enrolled between 2012 and 2017. These patients presented marked elevation of liver enzymes, and the rate of survival without LT was 81.6%. These patients were comprised of patients with ALI, ALF with or without coma, subacute liver failure, and LOHF. They had various etiologies of liver injury (hepatitis A virus 13%, hepatitis B virus 23%, autoimmune hepatitis 13%, drug-induced liver injury 8%, alcoholic liver injury [ALC] 11%, others 12%, unknown etiologies [UK] 20%, Table 1.
| Factor | Acute Liver Injury (n = 163) |
|---|---|
| Age (y) | 47 (38 - 61) |
| Gender (male/female) | 92/71 |
| Survival without LT (%) | 81.6 |
| Alb (g/dL) | 3.4 (3.0 - 3.8) |
| T-Bil (mg/dL) | 4.4 (2.5 - 10.8) |
| ALT (IU/L) | 1795 (565 - 4273) |
| LDH (IU/L) | 687 (380 - 2957) |
| ALP (IU/L) | 442 (327 - 621) |
| NH3 (μg/dL) | 66 (50 - 94) |
| BUN (mg/dL) | 12 (8 - 18) |
| Cr (mg/dL) | 0.73 (0.59 - 1.02) |
| Ferritin (ng/mL) | 3195.4 (704.13 - 11034) |
| WBC (/mm3) | 6480 (4760 - 9430) |
| Hb (g/dL) | 13.35(11.58 - 14.73) |
| Plt (/mm3) | 14.1 (9.6 - 18.7) |
| PT (%) | 43 (31 - 60) |
| FDP (μg/mL) | 12.1 (4.4 - 24.2) |
| Severity (ALI/ ALF without coma/ ALF with coma/ SALF/ LOHF) | 50/88/17/2/6 |
| Etiologies (HAV/HBV/AIH/DILI/ ALC/Others/UK) | 21/38/22/13/18/19/32 |
Abbreviations: LT, liver transplantation; Alb, albumin; T-Bil, total bilirubin; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; BUN, blood urea nitrogen; Cre, creatinine; WBC, white blood cell; Hb, hemoglobin; Plt, platelet; PT%, prothrombin activity percentage; FDP, fibrin degradation products; ALI, acute liver injury; ALF, acute liver failure; SALF, subacute liver failure; LOHF, late-onset hepatic failure; HAV, hepatitis A virus; HBV, hepatitis B virus; AIH, autoimmune hepatitis; DILI, drug-induced liver injury; ALC, alcoholic liver injury; UK, unknown etiology.
a Data are expressed as median and interquartile ranges.
4.2. ALI Was Often Accompanied by Elevated Pancreatic Enzymes
Of the 163 patients with ALI, 105 (64.4%) presented elevated pancreatic enzymes above the upper limit of normal, and 75 (54.0%) presented elevated levels above 1.5 times the upper limit of normal. Moreover, computed tomography (CT) imaging findings associated with pancreatitis were observed in 29 patients (17.8%). Edematous pancreatitis was observed in 13 patients (8.0%), and inflammatory changes in peripancreatic fat were observed in 25 patients (15.3%). Pancreatic necrosis was not observed in these patients (Table 2).
| Factor | Acute Liver Injury (n = 163) |
|---|---|
| Elevation of pancreatic enzyme (AMY or LIP) | |
| ≥ ULN | 105 (64.4) |
| ≥ ULN × 1.5 | 75 (54.0) |
| CT imaging findings associated with pancreatitis | 29 (17.8) |
| Edematous pancreatitis | 13 (8.0) |
| Inflammatory changes in peripancreatic fat | 25 (15.3) |
| Pancreatic necrosis | 0 (0) |
Abbreviations: AMY, amylase; LIP, lipase; ULN, upper limit of normal.
a Values are expressed as No. (%).
4.3. Elevation of Pancreatic Enzymes Was Associated with ALI Severity
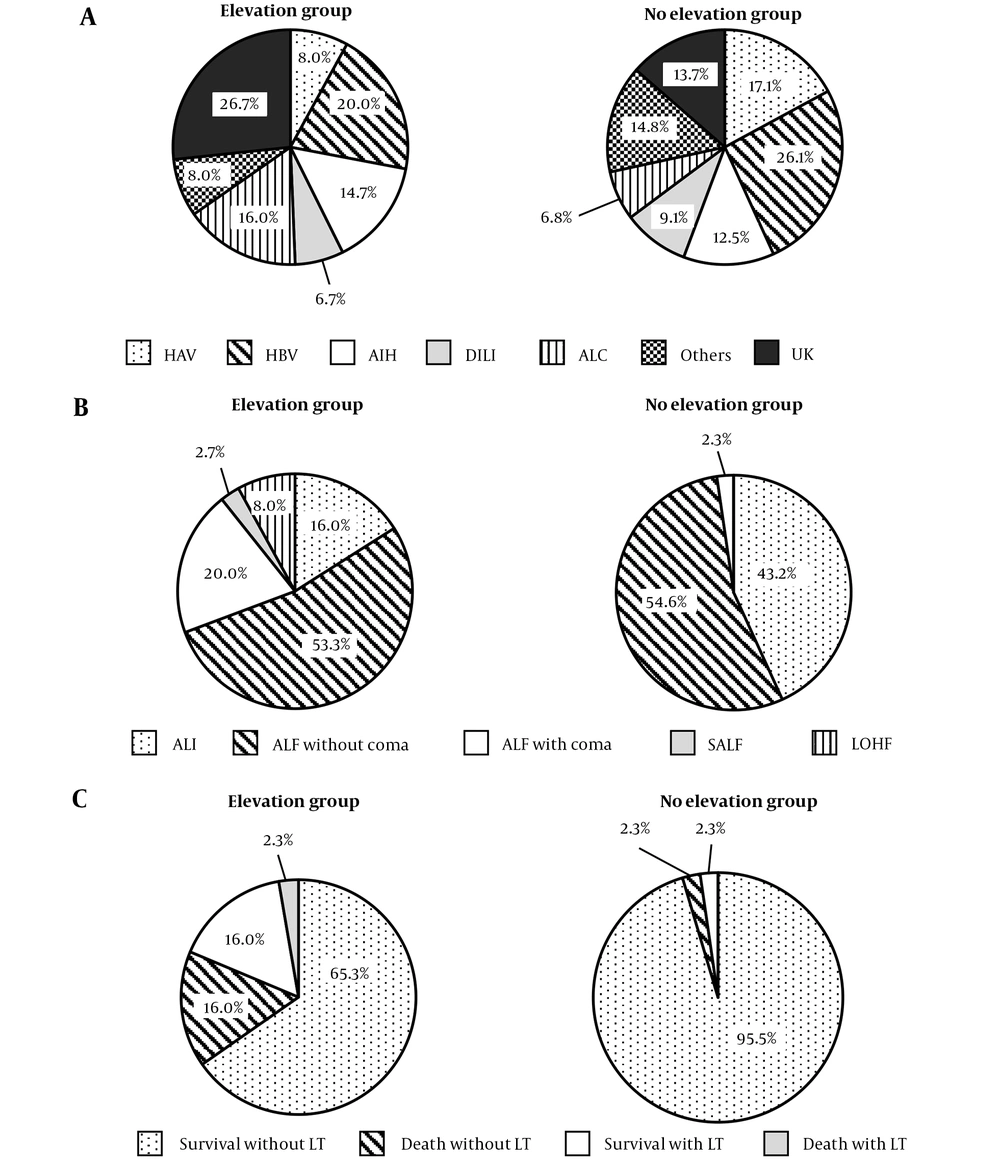
To evaluate the elevation of pancreatic enzymes accompanied by ALI, we compared patients with elevated pancreatic enzymes above 1.5 times the upper limit of normal (elevation group) to those without elevation (no elevation group). Although the elevation group tended to contain more cases of ALC and UK, the etiologies of the two groups were comparable (P = 0.0785, Figure 1A). The patients in the elevation group showed higher levels of total bilirubin, blood urea nitrogen, and creatinine; lower levels of albumin, platelet, and PT%; and a higher proportion of coma and ascites (Table 3). The elevation group was composed of the patients with more severe ALI states (Figure 1B). Furthermore, the patients in the elevation group had significantly poorer prognosis (Figure 1C). Because etiologies also correlated with severity and prognosis, it is possible that the etiologies affected the elevation of pancreatic enzymes accompanied by severity or prognosis. To exclude this possibility, we performed comparison of the etiologies between the elevation group and no elevation group in patients with ALI and ALF without coma. In both subgroups, the etiologies were not different between elevation group and no elevation group. In the ALF with coma, subacute ALF and LOHF, we could not perform subgroup analysis because of small sample size. We also divided patients into two subgroups based on whether patients survived without LT or not, and performed the same comparison. The association between etiologies and pancreatic enzymes was not detected in subgroup analysis. Thus, we considered that the etiologies did not affect the elevation of pancreatic enzymes.
Comparison between the elevation and no elevation groups. Comparison of the etiologies (A), severities (B), and prognosis (C), of ALI. χ2 test was used to assess significant differences between groups, and P-values were 0.0654, < 0.0001, and < 0.0001, respectively. HAV, hepatitis A virus; HBV, hepatitis B virus; AIH, autoimmune hepatitis; DILI, drug-induced liver injury; ALC, alcoholic liver injury; UK, unknown etiology; ALI, acute liver injury; ALF, acute liver failure; SALF, subacute liver failure; LOHF, late-onset hepatic failure; LT, liver transplantation.
| Factor | Elevation Group (≥ ULN × 1.5, n = 75) | No Elevation Group (< ULN × 1.5, n = 88) | P-Value |
|---|---|---|---|
| Age (y) | 50 (40 - 61) | 43 (35 - 59) | 0.034 |
| Gender (male/female) | 42/33 | 50/38 | 0.916 |
| Alb (g/dL) | 3.2 (3.0 - 3.8) | 3.6 (3.2 - 4.0) | 0.032 |
| T-Bil (mg/dL) | 6.8 (3.4 - 15.2) | 3.7 (1.9 - 7.1) | 0.0004 |
| ALT (IU/L) | 1313 (319 - 3801) | 2769 (696 - 4456) | 0.167 |
| LDH (IU/L) | 573 (380 - 2394) | 851 (382 - 3016) | 0.906 |
| ALP (IU/L) | 422 (308 - 608) | 454 (336 - 646) | 0.457 |
| NH3 (μg/dL) | 77 (57 - 115) | 60 (47 - 79) | 0.003 |
| BUN (mg/dL) | 15 (8 - 28) | 11 (8 - 16) | 0.0005 |
| Cr (mg/dL) | 0.93 (0.63 - 1.63) | 0.68 (0.58 - 0.83) | < 0.0001 |
| Ferritin (ng/mL) | 3360 (531 - 14921) | 2300 (880 - 8221) | 0.145 |
| WBC (/mm3) | 7710 (4760 - 12210) | 6190 (4763 - 8358) | 0.059 |
| Hb (g/dL) | 13.2 (10.4 - 14.7) | 13.8 (12.0 - 14.8) | 0.469 |
| Plt (/mm3) | 12.4 (7.8 - 17.2) | 15.0 (11.6 - 20.3) | 0.0025 |
| PT (%) | 36 (26 - 47) | 48 (37 - 69) | 0.0001 |
| FDP (μg/mL) | 14.4 (5.1 - 31.6) | 10.8 (3.9 - 19.1) | 0.198 |
| Proportion of patients with coma | 28.00% | 3.40% | < 0.0001 |
| Proportion of patients with ascites | 60.90% | 14.80% | 0.0011 |
| Flow volume of portal vein (mL/min) | 1095 (745 - 1433) | 1122 (788 - 1619) | 0.375 |
Abbreviations: Alb, albumin; T-Bil, total bilirubin; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; BUN, blood urea nitrogen; Cre, creatinine; WBC, white blood cell; Hb, hemoglobin; Plt, platelet; PT%, prothrombin activity percentage; FDP, fibrin degradation products.
a Data are expressed as median and interquartile ranges.
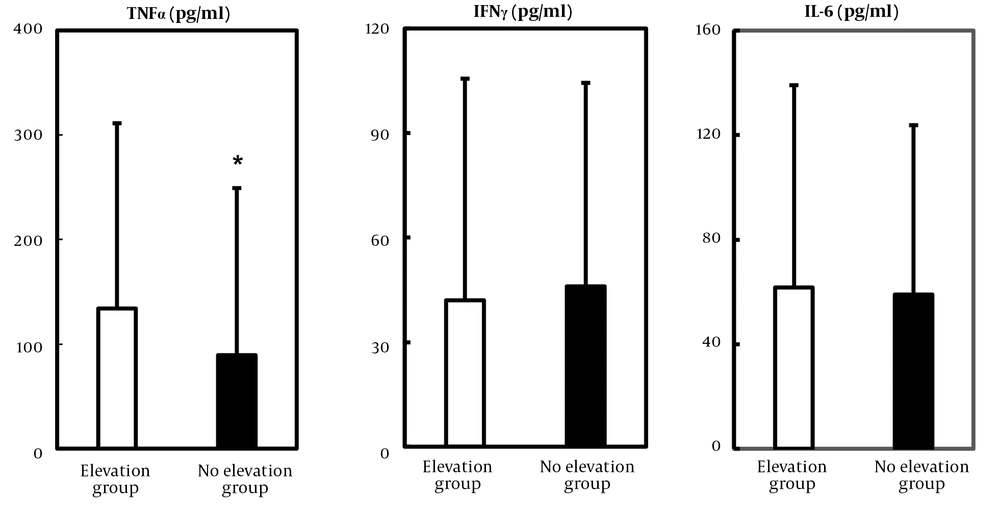
4.4. High Level of TNF-α Was Involved in the Elevation of Pancreatic Enzymes
We measured the serum levels of inflammatory cytokines to clarify the linkage between the elevation of pancreatic enzymes and ALI. Given that the serum TNF-α, IFN-γ, and IL-6 concentrations in healthy volunteers were reported to be less than 1.0 pg/mL (7), these cytokines were markedly elevated in both elevation and no elevation groups. Notably, TNF-α level was significantly higher in elevation group than no elevation group (elevation group Vs. no elevation group: 134.0 ± 177.2 pg/mL Vs. 89.4 ± 159.8 pg/mL, Figure 2).
5. Discussion
In this study, we showed that the elevation of pancreatic enzymes was associated with ALI severity and prognosis. Moreover, serum TNF-α levels correlated with the elevation of pancreatic enzymes in ALI patients. Acute pancreatitis often occurs in patients with ALI (1-3). Since ALI is often accompanied by renal failure, which influences the measurement of pancreatic enzymes, it is possible that the elevation of pancreatic enzymes is due to reduced renal excretion. However, Ham and Fitzpatrick reported that 33% of patients with ALF had autopsy-confirmed acute pancreatitis (1), and Ede et al. reported that the analysis of amylase isozyme avoided the influence of renal failure and revealed the high frequency of pancreatitis (34% of patients with ALF) (2). Moreover, some patients with normal renal function had elevated pancreatic enzymes, and a subset of patients presented CT imaging findings associated with pancreatitis in our analysis. Thus, we considered that these patients had bona fide pancreatic disorder.
The association between the elevation of pancreatic enzymes and ALI remains unknown. Some studies reported the possibility of a direct viral effect on acinar cells (8, 9). However, nonviral fulminant hepatic failure accompanied by pancreatitis has been reported (2). Moreover, in our analysis, we did not find differences in etiologies, and patients with nonviral liver injury presented elevated pancreatic enzymes similar to those with viral hepatitis. Thus, we considered that the elevation of pancreatic enzymes was the common event of viral and nonviral liver injury, and the direct viral effect on acinar cells was not the cause of elevated pancreatic enzymes. ALI is known to be associated with intrahepatic microcirculatory disorder (10). Intrahepatic microcirculatory disorder may influence the portal vein flow, and portal vein occlusion was reported to cause pancreatic inflammation in an experimental animal model (11). We evaluated the flow volume of the portal vein to clarify the involvement of hepatic microcirculatory disorder and found no differences between the elevation and no elevation groups. Thus, we considered that the microcirculatory disorder was not involved in the elevation of pancreatic enzymes in patients with ALI. Finally, we evaluated the cytokines in these patients. We found that the high level of TNF-α was associated with elevated pancreatic enzymes in patients with ALI. TNF-α was reported to be a prognostic marker of acute pancreatitis (12). Moreover, Sendler et al. reported that TNF-α directly activated premature protease and caused necrosis of pancreatic acinar cells in vitro (13). In some patients, the elevation of pancreatic enzymes was delayed after the development of ALI. Hence, we considered that the activation of inflammatory cells induced by massive liver destruction led to the secretion of TNF-α, resulting in pancreatic disorder. This may be the reason why the elevation of pancreatic enzymes was associated with renal failure, severity of liver damage, and poorer prognosis.
The influences of pancreatic disorder on ALI pathogenesis and mortality are controversial. Ede et al. reported that the pancreatic complication of ALF did not influence mortality (2); however, Kuo et al. reported that acute pancreatitis increased ALF mortality (14). In this study, although the CT imaging findings of the pancreas were mild, patients with ALI and elevated pancreatic enzymes had a poor prognosis. Since the elevation of pancreatic enzymes was associated with coagulopathy, renal dysfunction, and ALI severity, we considered that the pancreatic disorder reflected ALI severity, consequently correlated with mortality, and did not directly aggravate ALI pathogenesis.
In conclusion, we showed that elevated pancreatic enzymes often occurred in ALI and were associated with the severity of ALI. Furthermore, the elevated pancreatic enzymes were mediated by TNF-α signaling. Our analysis had certain limitations, such as the retrospective nature of the study, and further investigations are needed to verify our findings. Nevertheless, these findings could provide novel insights into the pathogenesis of ALI.