1. Background
The epidemiology of Hepatitis B (HBV) and C (HCV) remains poorly documented in Malaysia. Available data on the number of people who have chronic HBV and positive anti-HCV Abs (1-13) summarized in Tables 1 and 2 below, are based on mostly small studies in unrepresentative populations such as from a single institution (e.g., university, hospital), a single convenient group (e.g., blood donors, students, mothers) or at risk group (e.g., health workers, prisoners, people who inject drugs (PWID) or patients with chronic liver disease or on hemodialysis).
| Authors (Reference) | Year | Population | % HBsAg+ |
|---|---|---|---|
| Ton et al. (1) | 1979 | Male blood donors | 5.5% in all ethnic groups |
| Ton et al. (2) | 1980 | Prisoners | No data in abstract |
| Tan et al. (3) | 1989 | 2986 Healthcare employees | 2.1% (Chinese 6.3%, Malays 1.8%, Indian 0.9%) |
| Gan et al. (4) | 1991 | STD patients | 9.2% for multiple partners 6.8% for without multiple partners |
| Ng et al. (5) | 2005-2011 | 2923 newly enrolled university students | 0.62% (1.08% those born before 1989 and 0.20% born after 1989) |
| Ng et al. (6) | 1997-2003 | 190,077 school children aged 7-12 years | 2.5% for children born in 1985 to 0.4% among school children born in 1996. |
| Cheang et al. (7) | 2002-2010 | 440 mothers | 3.2% |
| Yousuf et al. (8) | 2007 | Blood donors | 1.83% |
| Authors | Year | Population | % Positive Anti-HCV Ab |
|---|---|---|---|
| Duraisamy et al. (9) | 1991- 1992 | Blood donors | Blood donors 1.5% |
| Ng et al. (10) | 1991 - 1992 | Blood donors; PWID; Patients with liver disease or on hemodialysis | Blood donors 1.9%; PWID 30%; Chronic liver disease 8%; Hemodialysis 9.1% |
| Sinniah and Ooi (11) | 1992 | Blood donors; PWID; Chronic liver disease patients | Blood donors 3%; PWID 85%; Chronic liver disease 1.5% |
| Lee and Ng (12) | 1999 - 2000 | Children aged 1 to 16 admitted in a hospital | 0.6% |
| Haslina et al. (13) | 2008 - 2009 | Blood donors | 0.14% (9/6495) |
2. Objectives
In this study, we provide estimates of the proportion of people who are positive for HBs Antigen (HBsAg) and anti-HCV Ab among participants in a community screening campaign.
3. Methods
The study sample population for this study is the people who attended the screening campaign organized by the Hepatitis Free Pahang (HFP). The Ministry of Health’s (MOH) Medical and Research Ethics Committee approved the study and all subjects gave oral informed consent. Between 2018 and 2019, HFP organized a total of 109 health fairs to conduct screening, mostly in small towns and villages and largely in the state of Pahang. All attendees at these health fairs registered online to participate in the screening tests. The online data system helped to support the conduct of the screening and administer questionnaire, manage screen-positive subjects for subsequent testing and counseling, facilitate reporting of results through short messaging service, and capture the data for this study.
To screen HBsAg and anti-HCV Ab, we used a low-cost point-of-care test (POCT, AllTest Biotech) that we have previously validated (14). The tests were conducted by a trained nurse. The procedure was explained, and verbal permission was obtained from the participant prior to the testing. Finger-stick capillary samples were taken from participants, and the tests were performed according to the manufacturer’s instructions. In the event of an invalid result, the test was repeated until a valid result was obtained.
All screened positive subjects were subsequently recalled, and 97% returned to undergo confirmatory testing, which were lab-based serologic tests (enzyme-linked immunosorbent assay) and nucleic acid tests for HCV RNA and HBV DNA (Real-time Polymerase Chain Reaction). A trained nurse counseled patients who had a confirmed chronic HBV, or positive anti-HCV Ab on infection transmission, had a risk of liver disease progression, and need for monitoring and treatment. Patients with confirmed chronic HBV, or positive anti-HCV Ab, were also referred to the local health service for further care. Also, HFP funded the direct-acting antiviral drugs for some indigent patients with positive anti-HCV Abs.
3.1. Statistical Methods
The sample size was based on an expected proportion of 2.0% HBsAg+ and precision of the estimate as measured by its 95% exact binomial confidence intervals (CI). For a sample size of 10,000, a proportion of 2.0% can be estimated with a 95% CI of 1.7 to 2.3, which is deemed sufficiently precise. Participants in the screening campaign constituted a convenient sample that was not representative of the general population (female, older subjects, and Chinese were over-represented compared to the population). To estimate the proportion of people who were positive for HBsAg and anti-HCV Ab among participants in the screening campaign, post-stratification (15) was used to adjust the total samples to known population totals for age, gender, and ethnicity based on the Population and Housing Census of Malaysia in 2010. We have undertaken a separate validation study of the POCT used in the screening. Using lab-based serological tests as the diagnostic standard, we determined the POCT for anti-HCV Ab had 98.1% sensitivity and 100% specificity, while that for HBsAg had 95.2% sensitivity and 100% specificity (14). We use these results to estimate the proportion of people who are positive for HBsAg and anti-HCV Ab among participants in the screening campaign, which are corrected for misclassification due to the use of the POCT screening tests (16).
4. Results
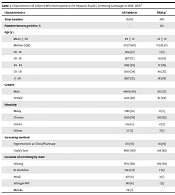
A total of 10,912 subjects participated in the hepatitis screening campaign and had screening tests in 2018 and 2019. We could not determine the number of subjects who attended the campaign but did not participate in the screening; hence, we could not estimate the response rate. Table 3 shows the characteristics of all the participants as well as the characteristics of the subjects who were screened positive for HBsAg or anti-HCV Ab. The mean age of the participants was 49 years, there were more female and older subjects, and Chinese were over-represented in the sample. The vast majority of subjects were screened at health fairs organized by local Non-Governmental Organizations (NGO) partners in the state of Pahang.
We estimated that 1.17% of adults aged 20 or older who participated in the screening campaign were positive for HBsAg+, and only 0.71 percent were positive for anti- HCV- Ab+ (Table 4). Young adults below 30 years of age had a very low proportion of HBsAg+ (0.09%). Proportion of subjects who were HBsAg+ or anti-HCV Ab+ increased with age, and then declined in the oldest age group (age ≥ 55 years). Men had a much higher proportion of HBsAg+ and anti-HCV Ab+ than women. Chinese had the highest proportion of HBsAg+, while Malay had the highest proportion of anti-HCV Ab+.
| Characteristics | All Subjects | HBsAg+ | Anti-HCV Ab+ |
|---|---|---|---|
| Total number | 10,912 | 200 | 52 |
| Number known positive, % | - | 145 | 15 |
| Age (y) | |||
| Mean ± SD | 49 ± 15 | 52 ± 12 | 49 ± 11 |
| Median (IQR) | 50 (37,60) | 51 (42,61) | 50 (40,58) |
| 20 - 30 | 1416 (13) | 3 (2) | 3 (6) |
| 30 - 39 | 1877 (17) | 36 (18) | 10 (19) |
| 40 - 49 | 2182 (20) | 57 (28) | 13 (25) |
| 50 - 59 | 2610 (24) | 46 (23) | 15 (28) |
| ≥ 60 | 2827 (25) | 58 (29) | 11 (21) |
| Gender | |||
| Male | 4400 (40) | 103 (51) | 28 (54) |
| Female | 6512 (60) | 97 (49) | 24 (45) |
| Ethnicity | |||
| Malay | 1782 (16) | 11 (5) | 20 (38) |
| Chinese | 8519 (78) | 183 (92) | 30 (57) |
| Indian | 556 (5) | 4 (2) | 1 (2) |
| Others | 55 (1) | 2 (1) | 1 (2) |
| Screening method | |||
| Opportunistic at Clinic/Pharmacy | 1111 (10) | 36 (18) | 11 (21) |
| Health fairs | 9801 (90) | 164 (82) | 41 (79) |
| Location of screening by state | |||
| Pahang | 8715 (80) | 190 (94) | 44 (85) |
| N. Sembilan | 1263 (12) | 7 (4) | 4 (8) |
| Perak | 477 (4) | 2 (1) | 1 (2) |
| Selangor-WP | 383 (4) | 1 (1) | 3 (5) |
| Melaka | 74 (1) | - | - |
a Values are expressed as No. (%) unless otherwise indicated.
| HBsAg+, % | 95% CI | Anti-HCV Ab+, % | 95% CI | |
|---|---|---|---|---|
| All | 1.17 | 1.5 - 0.83 | 0.71 | 0.97 - 0.44 |
| By age (y) | ||||
| 20 - 30 | 0.09 | 0.28 - 0.03 | 0.13 | 0.5 - 0.03 |
| 30 - 40 | 1.81 | 3.18 - 1.02 | 0.58 | 1.24 - 0.27 |
| 40 - 54 | 1.59 | 2.35 - 1.06 | 1.56 | 2.6 - 0.93 |
| ≥ 55 | 1.37 | 2.23 - 0.83 | 0.58 | 1.44 - 0.23 |
| By gender | ||||
| Male | 1.49 | 2.18 - 1.02 | 0.92 | 1.5 - 0.57 |
| Female | 0.82 | 1.24 - 0.54 | 0.48 | 0.86 - 0.27 |
| By Ethnicity | ||||
| Malay | 0.74 | 1.33 - 0.4 | 1.16 | 1.81 - 0.74 |
| Chinese | 2.25 | 2.64 - 1.92 | 0.37 | 0.55 - 0.24 |
| Others | 0.98 | 2.34 - 0.41 | 0.23 | 0.95 - 0.06 |
| By age-gender (y) | ||||
| Male | ||||
| 20 - 30 | 0.18 | 0.55 - 0.06 | 0.05 | 0.37 - 0.01 |
| 30 - 40 | 2.12 | 4.62 - 0.96 | 0.78 | 2.06 - 0.3 |
| 40 - 54 | 2.07 | 3.47 - 1.2 | 2.41 | 4.38 - 1.3 |
| ≥ 55 | 1.85 | 3.41 - 1 | 0.41 | 1.77 - 0.09 |
| Female | ||||
| 20 - 30 | 0 | 0 - 0 | 0.2 | 1.07 - 0.04 |
| 30 - 40 | 1.46 | 3.17 - 0.66 | 0.35 | 1.1 - 0.11 |
| 40 - 54 | 1.07 | 1.73 - 0.66 | 0.67 | 1.63 - 0.28 |
| ≥ 55 | 0.89 | 1.99 - 0.4 | 0.74 | 2.36 - 0.23 |
5. Discussion
We found that 1.17% of Malaysian adults who participated in the screening campaign were HBsAg+, and only 0.71% were anti-HCV Ab+. Chronic HBV has long been endemic in the Asia-Pacific region, including in Malaysia, as found in this study, though the prevalence has declined in many countries since the advent of universal vaccination in the 1990s (17). Our estimate of 1.17% HBsAg+ in adults is consistent with recent estimates in adults from large population-based studies (as opposed to institution-based studies or studies on special populations such as blood donors, students, PWIDs) in other Asia-Pacific countries such as China (18), Korea (19), Thailand (20) and India (21). The exception is Mongolia (22), which reported a high prevalence of 10.6% in adults in a recent national serosurvey.
Positive anti-HCV Ab is highly endemic in Central Asia and Mediterranean but not in the Asia-Pacific region (17). However, few recent population-based studies on HBV have been conducted in Asia. Our low estimate of anti-HCV Ab+ proportion (0.71%) is consistent with the 0.4% reported in China (23), 0.94% in Thailand (24) and 0.87% in India (25). Similar to HBV, Mongolia has an exceptionally high prevalence (11.1%) of anti-HCV Ab+ subjects in the Asia-pacific region (26). Our study is one of the largest seroprevalence surveys ever conducted in Malaysia. The large sample size is necessary to provide more precise age-sex and ethnic-specific estimates. We found the males had higher proportion of HBsAg+ and anti-HCV Ab+, which is expected. Similarly, Chinese had a higher proportion of HBsAg+, while Malay had higher anti-HCV Ab+.
The age trend in the proportion HBsAg+ showed an inverted U shape. The lower proportion of HBsAg+ in the oldest age group could be explained by pre-mature mortality from progression to cirrhosis and hepatocellular carcinoma (17). The low proportion of HBsAg+ in young adults below 30 years of age is likely due to the protective effect of universal HBV vaccination, which has been introduced in Malaysia since 1989. However, 0.9% of young adults aged 20-30 were positive for HBsAg suggesting persistent perinatal transmission. Elimination of HBV infection as a public health threat requires a reduction in the prevalence of HBsAg+ subjects to less than 0.1% in children five years of age (27). This cannot be achieved through universal HBV immunization of newborns alone. Interventions are necessary to prevent mother-to-child transmission of HBV, including antiviral prophylaxis (27, 28). Antenatal screening for HBsAg, which has been discontinued in Malaysia, should be reinstated, and WHO recommendation on the use of antiviral prophylaxis in pregnancy should be implemented (27).
Our study had several limitations. First, the study subjects were not a probability sample and is not representative of the population. Hence, there were more female and older subjects than in the general population, Chinese and Pahang residents were over-represented as a result of the conduct of the campaign through local NGOs, most of which were local faith or ethnic-based organizations in Pahang. Post-stratification was required to adjust the sampling weight to reflect the age, sex and ethnicity distribution of Malaysia. Second, subjects known to have hepatitis may be more or less willing to participate in screening. This source of bias applies to probability samples as well. Such subjects may be more or less willing to consent to be tested in a probability sampling survey. However, the risk of this bias was reduced in our study by pooling the data from numerous (109) screening events or venues conducted in numerous rural and urban locations spread over a wide geographical region. Third, for operational and cost reasons, we have used a POCT for screening instead of lab-based serology tests. The POCT has been validated, and the proportion estimates reported here are corrected for the misclassification bias due to the use of POCT.