1. Context
Chronic hepatitis B (CHB) virus infection is a global concern of public health that imposes a heavy economic burden on society. The number of people testing positive for hepatitis B virus (HBV) surface antigen (HBsAg) in 2016 was estimated at 291,992,000 (1). The prognosis of CHB patients has improved significantly since the clinical application of nucleos(t)ide analogues (NAs). Indications used to initiate antiviral treatment have also been developed, but there is debate about whether CHB patients with normal alanine aminotransferase (ALT) levels should receive antiviral treatment.
According to 2017 European Association for the Study of the Liver (EASL) CHB management guidelines, patients with hepatitis B envelope antigen (HBeAg)-positive CHB infection and defined as persistently normal ALT Levels (PNALT) plus high HBV deoxyribonucleic acid (DNA) levels, who are > 30 years of age, should receive treatment. Antiviral treatment can be initiated for CHB patients with a family history of hepatocellular carcinoma (HCC) or cirrhosis and extrahepatic manifestations, even if typical treatment indications are not fulfilled (2). The 2015 Asian Pacific Association for the Study of the Liver (APASL) CHB management guidelines suggested that fibrosis should be noninvasively assessed in CHB patients with PNALT (3). When there was evidence of significant fibrosis of non-invasive tests, patients > 35 years of age or with an HCC family history or cirrhosis should receive a liver biopsy, and antiviral treatment should start if there is existing evidence of moderate to severe inflammation or significant fibrosis (3). However, none of the current CHB guidelines support direct treatment for CHB patients with PNALT but rather emphasize the necessity of a family history of HCC and cirrhosis or evidence of liver inflammation and fibrosis in guiding the initiation of antiviral therapy when patients have PNALT levels.
Indications for initiating antiviral therapy remain controversial. Jeng and Lok (4) proposed that HBV treatment indications should be widened if available new therapies can safely achieve HBsAg loss in most patients when finishing a particular course of treatment. Recent studies indicated a tendency to expand indications for antiviral treatment in new CHB guidelines primarily because (1) NAs, including entecavir (ETV), tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide (TAF), have low resistance rates (5-7); and (2) NAs therapy is more cost-effective than the past (8). One expert opinion in 2020 recommended a treatment indication for CHB patients with ALT levels < 1* upper limit of normal (ULN) with fibrosis (≥ F2) and necroinflammation (≥ A2) classification of liver histology (9). This is a precise expression for guiding antiviral treatment for CHB patients. However, it is hard to manage liver biopsy in clinical practice to fulfill CHB treatment indications.
So far, antiviral treatment for CHB patients with PNALT was not common for not fulfilling typical treatment indications. Starting from an antiviral therapeutic perspective may provide another insight into this controversy of whether to treat CHB patients with PNALT.
2. Objectives
We aimed to compare the antiviral efficacy of CHB patients with PNALT and raised ALT levels after receiving NAs therapy. A systematic literature review and meta-analysis was performed to evaluate the performance of serological tests after NAs therapy in the two groups.
3. Methods
3.1. Search Strategy
We performed a systematic literature search in PubMed and Web of Science to identify all studies published from 1990.01 to 2022.08 using the following keywords from the medical subject headings (MeSH) database: "Chronic hepatitis B" AND other key words "treatment," OR "lamivudine" OR "adefovir" OR "entecavir" OR "telbivudine" OR "tenofovir" AND "ALT" OR "pretreatment" OR "PNALT." A manual literature search for references in retrieved articles was performed to supplement the findings.
3.2. Eligibility Criteria
Study inclusion was based on the PICOS criteria (participants/disease, intervention/exposure, comparison/control, outcomes/endpoints, and study design). All studies published in English with full manuscripts were under consideration if they met the following inclusion criteria: (1) prospective cohort studies like randomized clinical trials (RCTs) or rigorously designed retrospective cohort or case-control studies; (2) studies on patients diagnosed with CHB according to current diagnostic criteria, excluding other viral hepatitis (HAV and HCV) and human immunodeficiency virus (HIV) co-infections; (3) studies on patients receiving oral NAs monotherapy; and (4) studies with at least one of the clinical outcomes: ALT normalization, HBeAg loss, HBeAg seroconversion, and undetected HBV DNA also defined as a viral response. The provision of pre-treatment ALT levels was necessary.
3.3. Selection Process and Data Extraction
Two authors (Q.Z and H.M) performed the literature search independently and determined which studies met the inclusion criteria. The process was carried out under the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. Two trained physicians verified the results and evaluated the quality of the studies. After a discussion with the researchers, a final list of included studies was developed, and two authors extracted the data. Study details were extracted: Authors, study design, regions or countries, primary study questions, the numbers of patients with different levels of ALT and their demographic and clinical parameters, whether the patients were treated-naïve, the type of NAs patients received and their following-up duration, and the proportion of patients achieving three outcomes in both groups with normal ALT (40 IU/L ≤) and raised ALT (> 40 IU/L) (3).
3.4. Quality Assessment
The quality of RCTs, controlled clinical trials (CCTs), and cohort studies was assessed according to the cochrane collaboration risk of bias tool (ROB), methodological index for non-randomized studies (MINORS), and Newcastle-Ottawa Scale (NOS), respectively. The assessment was performed with RevMan 5.4.1.
3.5. Study Outcomes
The CHB patients were divided into two groups: The control group with normal ALT levels (ALT ≤ 1*ULN) and the experimental group with raised ALT levels (ALT > 1*ULN). A 40 IU/L level was considered the upper limit of normal for ALT when there was a lack of definition of ULN (3). The pooled effects were assessed for three outcomes: Viral response, HBeAg loss, and HBeAg seroconversion. The viral response was defined as serum HBV DNA below the lower limit of polymerase chain reaction (PCR) detection; however, each study used different detection thresholds and methods to assess the lower limit of HBV DNA detection (3). HBeAg loss was defined as the disappearance of HBeAg in patients with previous positive HBeAg (3), while HBeAg seroconversion was defined as the loss of HBeAg and detection of anti-HBe in serum samples at the same time in patients with previously positive HBeAg and negative anti-HBe (3). Patients were grouped based on ALT levels, as shown in Table 1, except those in one study (10) with an ALT cutoff of 1.5* ULN. Also, NAs were divided into first-line and second-line therapy for subgroup analysis. The first-line medicines included ETV, TDF, and TAF. The second-line medicines included lamivudine (3-TC), telbivudine (TBV), and adefovir (ADV) (11).
| Reference | Study Design | Country or Region | Primary Study Question | Definition of Normal ALT | Total Patients (n) | Patients with Normal ALT | Patients with Minimally Raised ALT | Patients with Raised ALT | Use of NAs | Parameters of outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Alexander et al. (12) | Not defined | India | To study the effect of Lamivudine on HBeAg loss and seroconversion rates in Indian patients of CHB | Not defined | 60 | 10 | 20 | 30 | Lamivudine | 1. HBeAg loss; 2. HBeAg seroconversion |
| Aung et al. (10) | RE; SC | Thailand, Southeast Asia | To compare the composite treatment outcome between CHB patients with low and high treatment-naïve viral load | < 40 IU/L | 95 | ALT < 1.5-time ULN:41; ALT ≥ 1.5-time ULN: 54 | Lamivudine; adefovir; entecavir; telbivudine | 1. ALT normalization; 2. HBeAg loss; 3. Undetectable HBV DNA | ||
| Bonino et al. (13) | PR; MC; RA | Multination but not specific | To report predictors of combined response to interferon and lamivudine | < 30 IU/L | 537 | - | 241 | 296 | Lamivudine | 1. ALT normalization; 2. HBV DNA level < 20 000 copies/mL |
| Chan et al. (14) | PR; MC; RA | Multinational but not specific | To evaluate treatment with either tenofovir disoproxil fumarate (TDF) and placebo or a combination of TDF and emtricitabine (FTC) for the treatment of patients with CHB | 43 U/L for male and 34 U/L for female | 126 | 60 | 0 | 0 | Tenofovir disoproxil fumarate | 1. ALT normalization; 2. HBeAg loss; 3. HBeAg seroconversion |
| Chien et al. (15) | RE; MC | Asian | To evaluate whether any factors influence HBeAg seroconversion | Not defined | 345 | 175 | 100 | Lamivudine | 1. HBeAg seroconversion | |
| Hann et al. (16) | RE; SC | Korea & America | To determine if the response to lamivudine treatment differs according to pre-therapy ALT levels. | Not defined | 317 | 102 | 96 | 119 | Lamivudine | 1. ALT normalization; 2. HBeAg loss; 3. Undetectable HBV DNA |
| Hom et al. (17) | PR; MC; RA | North America, South America, and Europe | To identify pretreatment factors predicting the likelihood of lamivudine response in children with chronic hepatitis B infection. | Not defined | 286 | 15 | 116 | 42 | Lamivudine | 1. HBeAg seroconversion; 2. Viral response |
| Jonas et al. (18) | PR; MC; RA | Asia, Europe, and America | To evaluate the safety and efficacy of entecavir for CHB in treatment-naive children and adolescents. | Not defined | 180 | 23 | 97 | Entecavir | 1. HBeAg seroconversion; 2. Viral response | |
| Kuo et al. (19) | PR; SC | The USA | To present results of 9 patients with lamivudine-resistant HBV treated with tenofovir disoproxil fumarate | Not defined | 9 | 1 | 2 | 6 | Tenofovir | 1. ALT normalization; 2. HBeAg loss; 3. HBeAg seroconversion; 4. Undectedable HBV DNA |
| Kwak et al. (20) | RE; MC | Korea | To evaluate the efficacy of 96 weeks of entecavir therapy in patients with resistance to lamivudine/adefovir sequential therapy | < 40 IU/L | 33 | 22 | 11 | Entecavir | 1. Undectedable HBV DNA | |
| Lee et al. (21) | RE; MC | Korea | To compare the antiviral efficacy of ADV and ETV after 52 weeks of treatment in patients with 3TC-resistant HBeAg- positive CHB | Not defined | 150 | 63 | 87 | Adefovir; entecavir | 1. Viral response | |
| Murray et al. (22) | PR; MC; RA | North America and Europe | To evaluate the safety and efficacy of tenofovir DF in adolescents with chronic hepatitis B | 43 U/L for male and 34 U/L for female | 106 | 29 | 77 | Tenofovir | 1. Undectedable HBV DNA | |
| Perrillo et al. (23) | PR; MC; RA | Asia, North America, Europe, Israel, South Africa, Australia, and New Zealand | To determine patient-dependent or laboratory variables that predict HBeAg loss | Not defined | 805 | 86 | 256 | 463 | Lamivudine | 1. HBeAg loss; 2. HBeAg seroconversion |
| Zhao et al. (24) | RE; MC | China | To determine the antiviral effect in CHB patients with ALT < 2 × ULN objectively | Not defined | 260 | 99 | 121 | - | Entecavir; lamivudine; telbivudine; adefovir; tenofovir; tenofovir alafenamide; fumarate | 1. Viral response; 2. HBeAg loss |
| Wu et al. (25) | RE; MC | Multinational | To evaluate the efficacy of Entecavir in chronic hepatitis B patients with mildly elevated alanine aminotransferase and; biopsy-proven histological damage | Not defined | 1347 | - | HBeAg(+): 190; HBeAg(-): 146 | HBeAg(+): 519; HBeAg(-): 492 | Entecavir | 1. ALT normalization; 2. HBeAg seroconversion; 3. Undectedable HBV DNA |
| Wu et al. (26) | RE; MC | China | To evaluate the frequency of fulfilling histological indication for antiviral therapy among chronic HBV patients with normal AL T | Not defined | 689 | 253 | 436 | Entecavir | 1. Undectedable HBV DNA; 2. HBeAg loss; 3. HBeAg seroconversion; 4. ALT normalization | |
Abbreviations: RE, retrospective; PR, prospective; SC, single-center; MC, multi-center; ALT, alanine aminotransferase; HBeAg, hepatitis B envelope antigen; ULN, upper limit of normal; HBV, hepatitis B virus; DNA, deoxyribonucleic acid.
3.6. Evaluation of Publication Bias
We conducted a funnel-plot analysis along with Egger's and Begg's tests to assess the publication bias in the studies. The data analysis was performed with Stata 17 software.
3.7. Data Statistics
A systematical meta-analysis was performed to evaluate the efficacy of NAs therapy among populations with elevated and normal ALT levels in three major clinical outcomes. The analysis was performed with Stata 17. The Mantel-Haenszel formula generated a pooled effect estimate with ORs and 95% confidence intervals (CIs). A fixed-effects model accounted for interstudy variability. Two-tailed t tests were used, and P < 0.05 was assumed statistically significant.
4. Results
4.1. Study Characteristics
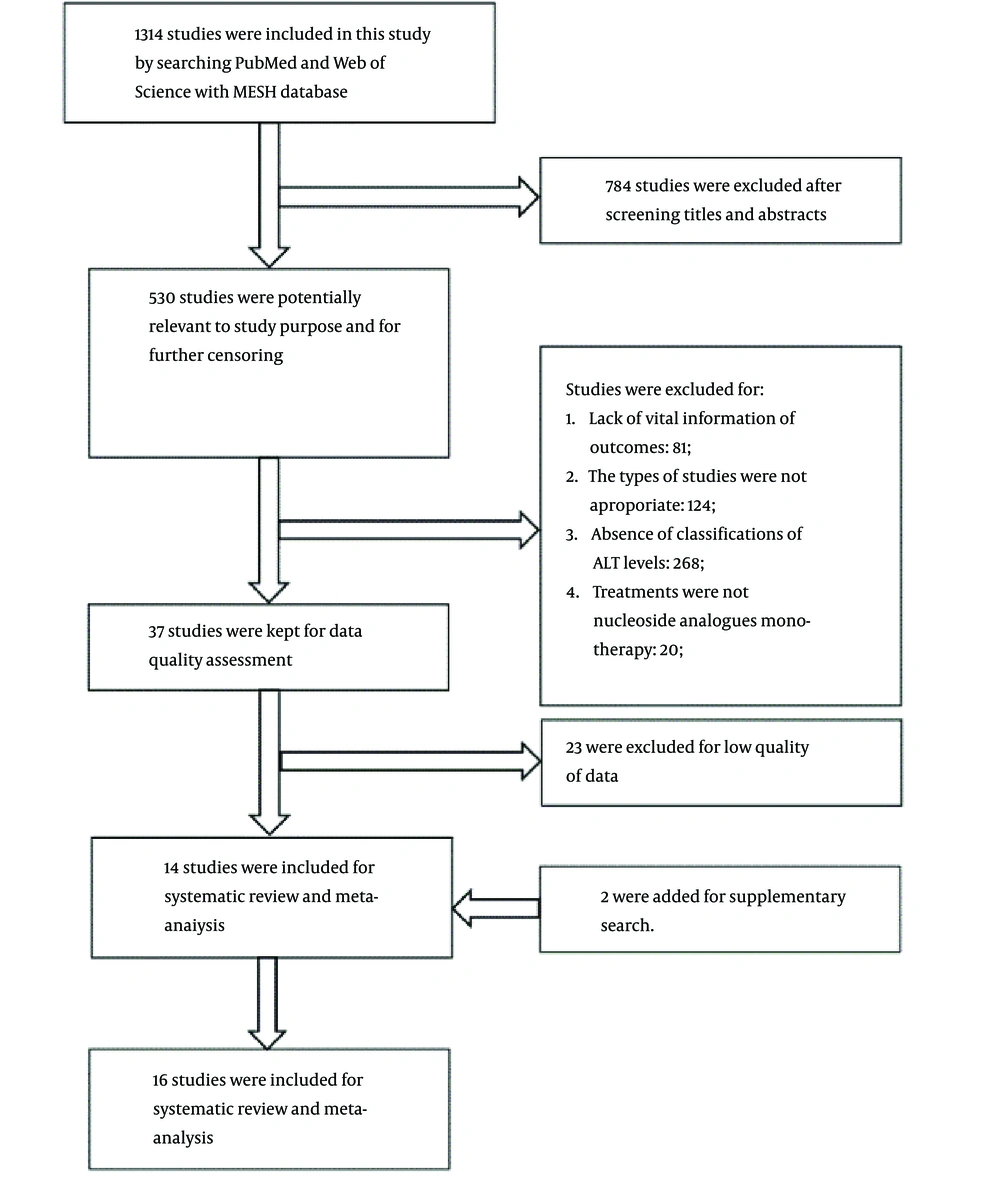
In an initial literature search, 1,314 studies were identified, of which 784 were excluded after the titles and abstracts were screened. Only 14 high-quality studies with adequate data collection remained, plus two studies added from the supplementary search for systematic review and meta-analysis (Figure 1). The 16 studies yielded 5,345 patients in total (10, 12-26). Of the studies, eight were retrospective cohort studies (10, 15, 16, 20, 24-27), and seven were prospective clinical trials (13, 14, 17-19, 22, 23). One study did not show the nature of the study (12). Twelve studies collected data from multi centers (13-15, 17, 18, 20-26), and five included a specific reference range for normal ALT levels (10, 13, 14, 20, 22). All the studies were evaluated as high quality (Appendix 1).
The patients were grouped into three based on their ALT levels: (1) the normal ALT group with ALT levels ≤ 1*ULN; (2) the minimally raised ALT group with ALT levels between 1*ULN and 2*ULN; and (3) the raised ALT group with ALT levels > 2*ULN. Of note, five studies only included patients with ALT levels < 2*ULN (13, 15, 18, 20, 21). Seven studies used lamivudine monotherapy as treatment regimens (10, 12, 13, 15-17, 23), four used entecavir (18, 20, 25, 26), two used tenofovir (19, 22), and two used TDF (14). Twelve studies provided data on viral response to assess clinical outcomes (10, 13, 16-22, 24-26), six of which could be used for further meta-analysis (16, 17, 19, 22, 24, 26). Eight studies provided data of HBeAg loss (10, 12, 14, 16, 19, 23-25), six of which could be used for meta-analysis (12, 16, 19, 23-25). Only nine studies provided data on HBeAg seroconversion (12, 14, 15, 17-19, 23, 25, 26), five of which (12, 17, 19, 23, 26) met the meta-analysis criteria (Table 1).
4.2. Patient Characteristics
Of the total patients, 4,806 were diagnosed with CHB, including 3,759 HBeAg(+) and 1047 HBeAg(-) patients. Patients had a mean or median age of 40 - 50 years in six studies (10, 12, 16, 19, 21, 24) and an age < 20 years in two studies (18, 22). Thirteen studies included the male-to-female ratio (10, 12, 14, 16, 18-26), which was 2344/856 combined across all studies. Ten of the 16 studies provided patients’ average ALT levels (10, 12, 14, 18-22, 24, 25), thirteen provided HBV DNA loads (10, 12, 14, 16, 18-26) and four (12, 16, 21, 23) provided the number of individuals with cirrhosis. Four studies also provided body mass index (BMI) (12, 14, 24, 26), and three studies provided Hepatitis Activity Index (HAI) scores (Table 2) (23, 25, 26).
| Reference | Patients (n) | Age a (y) | Gender (M/F) | Race | ALT (IU/L) | Serum HBV DNA | Cirrhosis (n) | BMI | HAI-Score | |
|---|---|---|---|---|---|---|---|---|---|---|
| HBeAg(+) | HBeAg(-) | |||||||||
| Alexander et al. (12) | 60 | 0 | 40 (4 - 80) a | 50/10 | Indian | 72 (27 - 394) a | 820 (0.8 - 4500) mEq/mL a | 23 | 22.8 (16.4 - 29.5) a | - |
| Aung et al. (10) | 41 | 54 | 44.5 ± 11.5 b | 69/26 | Thai | 64 (36 - 158) c | 6.38 (5.22 - 7.52) log10 copies/mL c | - | - | - |
| Bonino et al. (13) | 537 | 0 | - | - | - | - | - | - | - | - |
| Chan et al. (14) | 63 | 1 | 33 (18 - 62) a | 31/33 | Asian, white, black, Pacific islander, other | 26.9 ± 14.05 b | 8.42 (8.02 - 10.22) log10 IU/mL a | - | 23.5 (17.8 - 35.4) | - |
| Chien et al. (15) | 345 | 0 | - | - | Asian | - | - | - | - | - |
| Hann et al. (16) | 201 | 116 | HBeAg(+): 42.4 (16.6 - 78.3) a; HBeAg(-): 48.1 (18.7 - 68.3) a | 224/93 | Korean | - | HBV DNA (+): 240; HBV DNA (-): 35; Unknown: 42 | HBeAg(+): 59; HBeAg(-): 50 | - | - |
| Hom et al. (17) | 286 | 0 | - | - | - | - | - | - | - | - |
| Jonas et al. (18) | 180 | 0 | 10.5 (0.455) a | 78/42 | Asian, white, black | 107.1 (5.388) | 8.14 ± 0.0892 log10 IU/mL b | - | - | - |
| Kuo et al. (19) | 9 | 0 | 48.7 d | 8/1 | Asian, African-American, Caucasian | 288 | 1.9*108 copies/mL d | - | - | - |
| Kwak et al. (20) | 27 | 5 | 51 ± 20 b | 29/4 | Asian | 646 ± 628 b | 6.46 ± 2.62 log10 copies/mL b | - | - | - |
| Lee et al. (21) | 150 | 0 | ADV group: 46 (19 - 75); a ETV group: 45 (21 - 79) a | ADV group:70/21; ETV group:44/15 | Asian | ADV group: 144 ± 100; b ETV group: 163 ± 166 b | ADV group: 7.33 ± 0.91 log10 copies/mL; b ETV group: 7.37 ± 0.96 log10 copies/mL b | ADV group:21; ETV group: 10 | - | - |
| Murray et al. (22) | 96 | 10 | 15.4 ± 1.38 (12, 17) e | 73/33 | White, Asian, black, other | 101 ± 98.5 (16, 563) e | 8.13 ± 1.4 (4.8, 10.1) log10 copies/mL e | - | - | |
| Perrillo et al. (23) | 805 | 0 | 34 (15 - 73) a | 316/90 | White, Asian, black, other | 2.2 (0.3 - 23.4) *ULN a | 98; LLOD-2,264 pg/mL | 36 | - | 10 (2 - 2) |
| Zhao et al. (24) | 119 | 101 | 40.05±0.70 | 185/75 | Asian | 59.55 ± 1.88 | 5.70 ± 0.11 log10 IU/mL | - | 23.42 ± 0.20 | - |
| Wu et al. (25) | 709 | 638 | HBeAg(+): 35 | HBeAg(+): 532/177 | Not supported | HBeAg(+): 143 | HBeAg(+): 9.7 log10 copies/mL | - | - | HBeAg(+): 8 |
| HBeAg(-): 44 | HBeAg(-): 485/153 | Not supported | HBeAg(-): 142 | HBeAg(-): 7.6 log10 copies/mL | - | - | HBeAg(-): 8 | |||
| Wu et al. (26) | 131 | 122 | 39.7±11.1 | 170/83 | Asian | 0.7 ± 0.2 ULN | 5.6 ± 2.1 log10 IU/mL | - | 23.1 ± 2.8 | 4.2 ± 2.1 |
Abbreviations: ULN, upper limit of normal; ETV, entecavir; ADV, adefovir; HBeAg, hepatitis B envelope antigen.
a Median + range.
b Mean ± SD.
c (Median, IQR).
d Mean.
e Mean ± SD (range).
In all studies, 3,687 patients were treated with NAs. Patients in eight studies were treated-naïve (10, 12, 14, 15, 18, 24-26). Patients in three studies had received NAs previously (19-21), and one study treated patients who had received both NAs and interferon previously (22). Three studies did not provide specific details of prior treatment regimens (13, 16, 17). Part of the patients in one study (23) had received NAs and interferon previously. The mean or median duration of follow-up ranged from 24 to 192 weeks (Table 3).
| Reference | Patients (n) Treated with NAs | Type of Patients | Characteristics of Therapy | Viral Response Using Different Definitions | HBeAg Loss | HBeAg Seroconversion | ALT Normalization | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial Treatment | Duration of Following-up | Normal ALT | Minimally Raised ALT | Raised ALT | Normal ALT | Minimally Raised ALT | Raised ALT | Normal ALT | Minimally Raised ALT | Raised ALT | Minimally Raised ALT | Raised ALT | |||
| Alexander et al. (12) | 60 | Treated naive | 3-TC | 3 years (156 weeks) | - | - | - | 3/10 | 10/20 | 22/30 | 0/10 | 6/20 | 18/30 | - | - |
| Aung et al. (10) | 95 | Treated naive | 3-TC; ADV; ETV; TBV | 1 year (52 weeks) | ALT < 1.5 time ULN; ALT ≥ 1.5 time ULN | ALT < 1.5 time ULN; ALT ≥ 1.5 time ULN | - | - | - | ALT < 1.5 time ULN | ALT ≥ 1.5 time ULN | ||||
| 21/41; 37/54 | - | - | - | ||||||||||||
| Bonino et al. (13) | 181 | Not specified | 3-TC | 24 weeks | 17/78 | 25/103 | - | - | - | - | - | - | 17/78 | 25/103 | |
| Chan et al. (14) | 64 | Treated-naive | TDF | 192 weeks | 29/64 | - | - | 4/63 | - | - | 3/63 | - | - | 41/64 | - |
| Chien et al. (15) | 275 | Treated-naive | 3-TC | 52 weeks | - | - | - | - | - | - | 6/175 | 33/100 | - | - | |
| Hann et al. (16) | 317 | Not specified | 3-TC | 36 months (144 weeks) | 34/40(+) | 42/48(+) | 68/73(+) | 45/57 | 48/58 | 73/86 | - | - | - | 52/58(+) | 82/86(+) |
| 22/27(-) | 22/28(-) | 22/24(-) | 33/38(-) | 30/33(-) | |||||||||||
| Hom et al. (17) | 191 | Not specified | 3-TC | 52 weeks | 1/8 | 10/86 | 33/97 | - | - | - | 1/8 | 11/86 | 36/97 | - | - |
| Jonas et al. (18) | 120 | Treated-naive | ETV | 96 weeks | 7/23 | 52/97 | - | - | - | 3/23 | 26/97 | - | - | ||
| Kuo et al. (19) | 9 | Treated with NAs before | TDF | 11.2 (6 - 16) months; (44.8 (24 - 64) weeks) | 1/1 | 2/2 | 4/6 | 0/1 | 0/2 | 3/6 | 0/1 | 0/2 | 2/6 | 0/2 | 2/6 |
| Kwak et al. (20) | 33 | Treated with NAs before | ETV | 48 - 96 weeks | 7/22 | 1/11 | - | - | - | - | - | - | - | - | |
| Lee et al. (21) | 150 | Treated with NAs before | ETV; ADV | 24 weeks | 6/28(E); 11/35(A) | 19/31(E); 29/56(A) | - | - | - | - | - | - | - | - | |
| Murray et al. (22) | 52 | Treated with NAs and Interferon before | TDF | 72 weeks | 12/17 | 34/35 | - | - | - | - | - | - | - | - | |
| Perrillo et al. (23) | 406 | Part of the patients was treated with NAs or Interferon before | 3-TC | 52 weeks | - | - | - | 2/55 | 18/120 | 82/231 | 1/53 | 8/114 | 57/224 | - | - |
| Zhao et al. (24) | 190 | Treated-naive | Combined NAs | 72 weeks | 73/79 | 98/111 | - | 4/40 | 2/61 | - | - | - | - | - | - |
| Wu et al. (25) | 1347 | Treated-naive | ETV | 48 weeks | - | 46/95(+); 69/80(-) | 190/259(+); 224/245(-) | - | - | - | - | 13/190(+) | 125/519(+) | 52/95(+); 61/80(-) | 190/259(+); 192/245(-) |
| 3-TC | 20/95(+); 47/66(-) | 109/260(+); 178/247(-) | 60/95(+); 45/66(-) | 153/260(+); 177/247(-) | |||||||||||
| Wu et al. (26) | 197 | Treated-naive | ETV | 78 weeks | 38/57 | 97/140 | 12/57 | 27/140 | 3/57 | 11/140 | - | - | |||
Abbreviations: ETV, entecavir; TDF, tenofovir disoproxil fumarate; TAF, tenofovir alafenamide; 3-TC, lamivudine; TBV, telbivudine; ADV, adefovir; ALT, alanine aminotransferase; HBeAg, hepatitis B envelope antigen; NAs, nucleos(t)ide analogues.
4.3. Comparison of Outcomes Between Two Groups
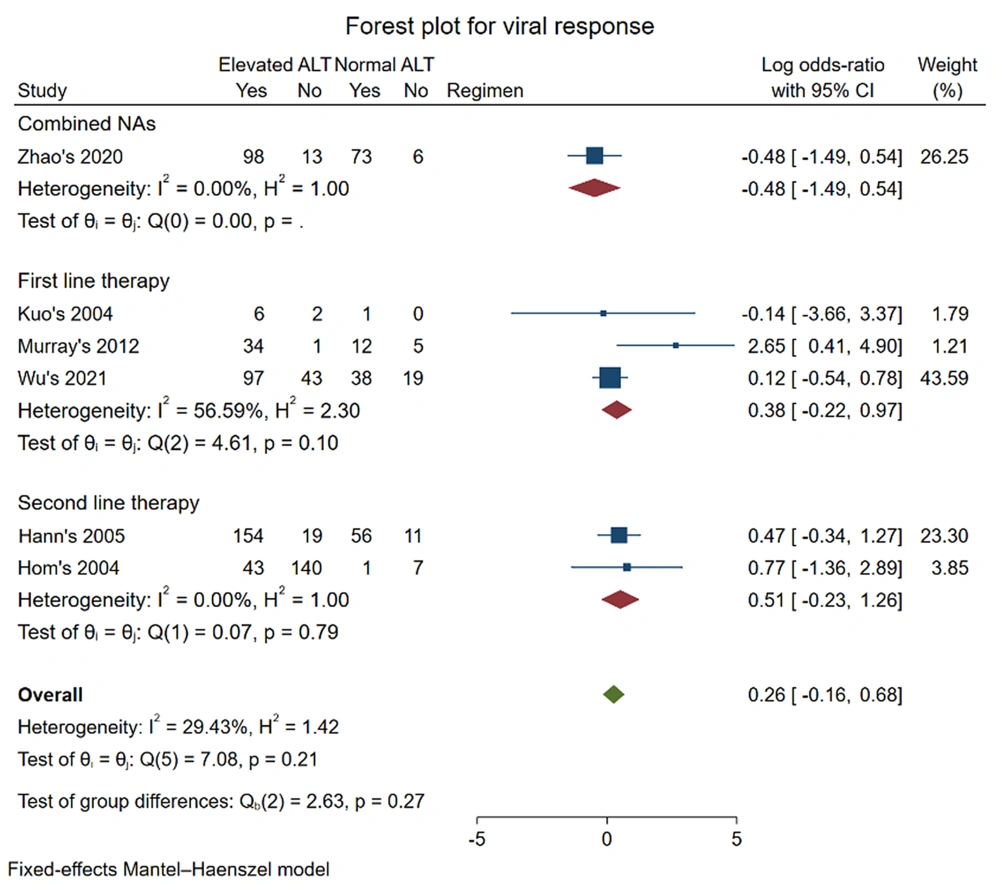
No statistically significant differences between the elevated and normal ALT groups were observed in the outcome of viral response and HBeAg loss for first-line therapy. For the outcome of viral response, six studies were included in the meta-analysis (16, 17, 19, 22, 24, 26). First-line therapy of TDF and ETV was adopted in three studies (19, 22, 26), and 3-TC was adopted in two studies (16, 17). One study used multiple NAs, including ETV, 3-TC, TBV, ADV, tenofovir, and TAF (24). The pooled log OR was (0.38 [-0.22, 0.97], P = 0.10) in first-line therapy and (0.26 [-0.16, 0.68], P = 0.27) for all regimens. According to our findings, whether pretreatment ALT level was above 1*ULN was not an indicator for predicting the viral response of NAs when both first-line therapy with TDF and ETV or second-line therapy with 3-TC were applied for CHB patients.
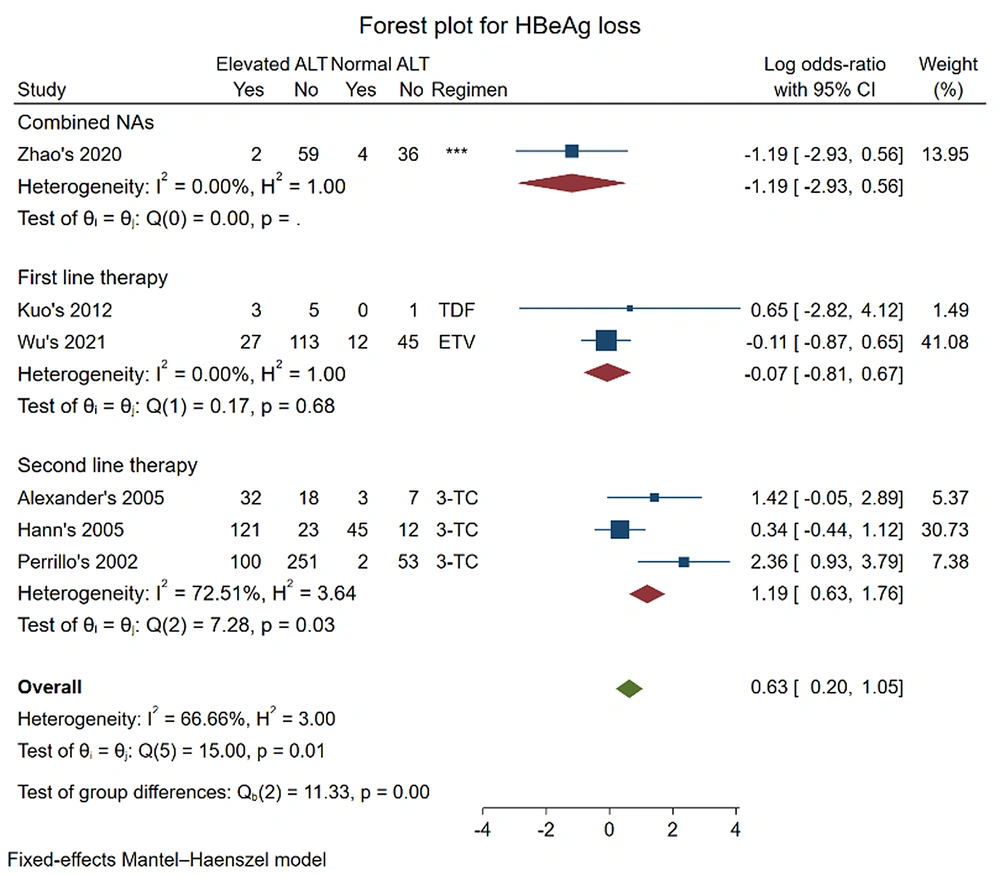
Six studies were included in the meta-analysis for the outcome of HBeAg loss (12, 16, 19, 23, 24, 26). The pooled log OR was (-0.07 [-0.81, 0.67], P = 0.68) in first-line therapy and (0.63 [0.20, 1.05], P = 0.01) for all regimens. The results showed no significant difference in HBeAg loss between the two groups of CHB patients who received first-line therapy with ETV and TDF. However, the result changed when the data of second-line therapy with 3-TC (pooled log OR: (1.19 [0.63, 1.76], P = 0.03)) were added in.
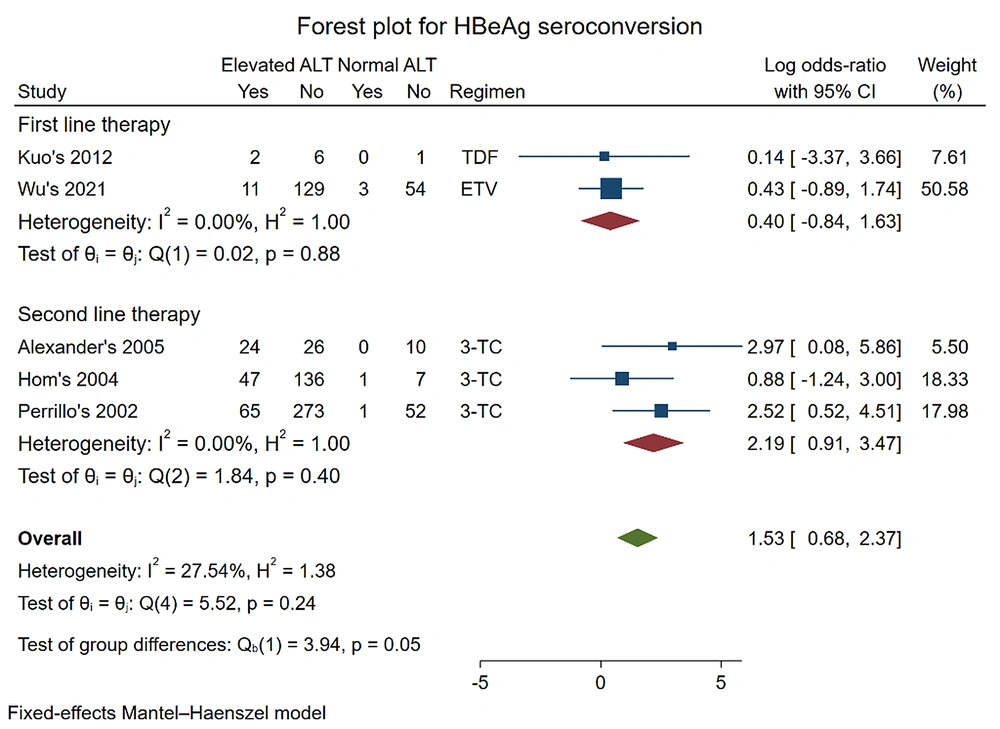
Five studies were included to analyze HBeAg seroconversion (12, 17, 19, 23, 26). The pooled log OR was (0.40 [-0.84, 1.63], P = 0.88) in first-line therapy with TDF and ETV and (2.19 [0.91, 3.47] P = 0.40) for second-line therapy with 3-TC. When the data were combined, the result did not change: Pooled log OR (1.53 [0.68, 2.37], P = 0.24) for all regimens.
4.4. Publication Bias
We conducted a funnel-plot analysis with Egger's and Begg's tests to assess the publication bias in the studies included. For viral response, the results showed no obvious publication bias (regression-based Egger’s test: Z = 1.09, probability > |Z| = 0.2746; Begg’s test: Z = 0.38, probability > |Z| = 0.7071) (Appendix 2). There was no evidence of publication bias for HBeAg loss (regression-based Egger’s test: Z = 1.01, probability > |Z| = 0.3146; Begg’s test: Z = 0.00, probability > |Z| = 1.0000) (Appendix 3) and HBeAg seroconversion (regression-based Egger’s test: Z = 0.93, probability > |Z| = 0.3537; Begg’s test: Z = -0.24, probability > |Z| = 1.0000) (Appendix 4).
5. Discussion
CHB patients with PNALT were not previously considered for antiviral treatment. This was because (1) liver biopsies from patients with an immunotolerant (IT) phenotype (defined as HBeAg positivity, PNALT, and HBV DNA > 107 cp/mL) showed only minimal changes (28) when PNALT was associated with a low incidence of histologically significant liver disease (29); (2) disease progression in this patient population was considered slow (30); and (3) the risk of developing HCC in IT-phase patients who were HBeAg(+) was low (27). A retrospective single-center study of medical records for patients with CHB concluded that widening treatment indications was unlikely to alter HCC incidence (31).
However, more recent studies have shown contradictory results about the significance of histological changes in CHB patients. One study (32) found that around 21% of HBeAg-negative CHB patients with persistently normal ALT levels and HBV DNA viral loads < 5 -log10 copies/mL had active histological liver disease (defined as histologic activity index > 3 and/or fibrosis stage > 2) through liver biopsy checks. These findings were confirmed by a subsequent study (33). Another retrospective report of 101 treated-naïve CHB patients found that CHB patients with high HBV DNA viral loads (≥ 10,000 copies/mL), normal ALT levels, and age < 35 years old had no or minimal histological disease, while CHB patients aged over 35 years more often had significant histological disease (34). Another study (35) found significant liver fibrosis and inflammation in 37% of CHB patients with PNALT. Another study reported similar findings that about 38.2% of HBeAg-negative CHB patients with low HBV DNA levels (< 2000 IU/mL) and PNALT had significant liver disease (36). However, there were still some controversies remaining. A study collected data from a public database and demonstrated that an ALT level < 2 times ULN is associated with a < 5% probability of significant inflammatory activity among CHB patients without significant fibrosis (37). These findings suggested that although ALT was a major determinant for indicating the treatment, there was not always consistency between ALT level and liver histological changes.
In addition to the inconsistent findings on pathological liver changes between CHB patient groups with elevated or normal ALT levels, there were controversies in the clinical benefits of antiviral therapy between the two groups. One study (38) published in 1981 showed that the incidence of primary HCC is considerably higher among HBsAg(+) than in HBsAg(-) patients (1158/100 000 vs. 5/100 000 over 75,000 person-years) in a prospective general population study of 22,707 Chinese adults. This study first indicated the clinical outcomes of CHB patients receiving no therapy. Chu et al. (39) followed 240 HBeAg(+) CHB patients with normal ALT levels for 17 years and found that the annual incidence of liver cirrhosis was 0.5%, and the 17 years' cumulative probability of cirrhosis was 12.6%, indicating that CHB infection in the IT phase may not be a benign disease and can result in adverse clinical outcomes. Another prospective study (40) followed 3,233 Chinese CHB patients. It concluded that prolonged low-level viremia causes insidious and continual liver damage, as reflected by ALT levels of 0.5-2*ULN, and is the most likely reason for the development of complications, such as liver cirrhosis and HCC, in CHB patients. Contrary to the above findings, HCC risk was low during the stringently defined untreated immune-tolerant phase of CHB patients (41).
However, the results may be different when these patients receive treatment. A retrospective cohort study (42) specifically concluded that untreated CHB patients in the immune-tolerant phase had a higher risk of HCC and death or a higher probability of liver transplantation than treated IT-phase patients. The same results were repeated later (43). These results implied the need to treat CHB patients with normal ALT levels, and the unnecessary mortality could be avoided by receiving earlier antiviral treatment for a particular group of IT-phase patients. These findings require the support of clinical data from prospective RCTs.
Several studies published after 2010 assessed the treatment efficacy of NAs in CHB patients with normal ALT levels. One study (25) evaluated the relationship between pretreatment ALT levels and treatment efficacy and showed that HBeAg-negative CHB patients responded similarly to those on entecavir, irrespective of baseline ALT levels. Another study enrolled 235 CHB patients with positive HBV DNA results and persistent normal or mildly elevated ALT levels and treated them with entecavir and adefovir. Antiviral therapy could improve or regress hepatic fibrosis and cirrhosis (44). However, for HBeAg-positive patients, those with ALT levels between 1 and 2*ULN responded less well to antiviral treatment than those with ALT > 2*ULN. Another study indicated whether pretreatment ALT levels were normal or not did not impact viral response and HBeAg loss outcomes after NAs therapy (26). This was consistent with our meta-analysis. Chan et al. (14) compared the efficacy of TDF monotherapy and TDF plus ETC combination therapy in IT-phase patients (defined as normal ALT and HBV DNA > 1.7*107 IU/mL), finding that both therapies could achieve viral suppression. Hoang et al. (45) further concluded that antiviral therapy could decrease the risk of progression to HCC in CHB patients having ALT levels of 1-2*ULN.
In our review, we found that when CHB patients were divided into normal and elevated ALT groups, receiving first-line therapy with ETV, TDF, and TAF, their response towards NAs evaluated as a viral response, HBeAg loss, and HBeAg seroconversion showed no significant difference. Furthermore, when the two groups received therapy with 3-TC, viral response and HBeAg seroconversion outcomes showed no between-group differences. Because of the high rates of drug-resistant mutations and treatment adverse events, 3-TC is no longer recommended as first-line therapy (46). The two groups of CHB patients can successfully achieve viral response whether receiving first-line or second-line NAs. The finding was consistent with the results of recently published studies (24, 26). Since HBV DNA viral load is strongly related to the development of HCC (47), the rates of HCC development are lower in CHB patients undergoing NAs therapy than in those without NAs therapy (48). In addition, the induction of HBeAg loss, with or without HBeAg seroconversion, in HBeAg-positive CHB patients is a valuable endpoint, as it often represents a partial immune control of chronic HBV infection (2). Accordingly, our analysis suggests that the results of NAs treatment in CHB patients with or without elevated ALT are similar. It is still unknown whether early NAs treatment for CHB patients with PNALT may reduce the occurrence of endpoint events like cirrhosis and HCC, which requires further research.
It is reasonable to expect our findings to provide insight into the rationale for widening CHB treatment indications.
5.1. Limitations
We acknowledge the following limitations of our study. First, the number of studies included was limited, so a publication bias may not be easily avoided, although it was assessed with multiple statistical methods. Moreover, the study population included was limited. Second, part of the included study population for evaluating viral response was a combination of both HBeAg-positive and HBeAg-negative CHB patients. Third, the specific value of the upper limit of normal for ALT levels was different in some studies, which may influence the study result.