1. Background
Hepatitis E, an infectious disease caused by the hepatitis E virus (HEV), is prevalent worldwide and has gained increasing attention (1, 2). While acute hepatitis E in industrialized nations rarely requires antiviral treatment, chronic HEV genotype 3 infection in immunosuppressed patients should be treated by reduction of immunosuppression or ribavirin as suggested by the EASL guidelines (3). Mutations in HEV polymerase have been associated with cases of ribavirin treatment failure (4, 5). In single cases without clearance of HEV infection under ribavirin treatment, antiviral treatment with interferon should be reflected (3). However, reduction of immunosuppression or use of interferon harbors the risk of rejection in transplant recipients, and ribavirin is associated with side effects, mainly anemia. As there are approximately 10% of patients with chronic hepatitis E not achieving sustained virological response (SVR) despite ribavirin therapy, novel antiviral strategies are urgently needed to cure difficult-to-treat patients (6, 7).
Zinc salts, widely used as dietary supplements, have been reported to have a broad spectrum of antiviral activity (e.g., against human immunodeficiency virus, Coronavirus, rhinovirus, respiratory syncytial virus, or herpes simplex virus) (8-11). Recently, zinc has been shown to have antiviral properties against HEV genotype 1 in the cell-cycle system (12). Furthermore, zinc serum levels are decreased in patients with viral hepatitis, including those with hepatitis E (13). Pathogenesis of zinc-mediated HEV inhibition has been suggested to include the release of interferon-alpha and interferon-gamma inhibiting HEV replication and a potential direct inhibitory effect of zinc on HEV RNA-dependent RNA polymerase (14). However, there are neither in vitro nor in vivo data regarding the efficacy of zinc supplementation for HEV genotype 3 infections.
2. Objectives
We aimed to study the role of zinc as a possible therapeutic approach in chronic HEV patients without achieving SVR under ribavirin monotherapy. For this purpose, the antiviral efficacy of zinc salts on HEV genotype 3 was analyzed in vitro, in an animal model, and human patients.
3. Methods
3.1. HEV-Replicon Model
The antiviral properties of zinc salts have been studied in Huh-7.5 cell lines expressing a subgenomic replicon of HEV3 (Kernow) or HEV1 (Sar55). MTT assays determined cell viability. Magnesium served as a negative control. These experiments were performed at the Hannover Medical School in line with previously described methods (15).
3.2. HEV-Rabbit Model
Twenty-four 3-month-old Japanese white rabbits weighing 2.0 and 3.0 kg were randomly divided into 4 groups (A-D). Animals were separately kept in independent cages, and adequate food and water were fed. Before the experiment, fecal and serum samples from all animals were collected to ensure negative for HEV RNA for 2 weeks by real-time quantitative PCR (RT-qPCR). All serum samples were tested negative for anti-HEV antibodies by enzyme-linked immunosorbent assay (ELISA). The virus strain used in this study was recovered from fecal samples of a farmed rabbit in Beijing (CHN-BJ-R14, genotype 3, GenBank JX109834). The inoculum was tested by RT-qPCR and showed a 106 copies/mL level for HEV RNA. All animals were inoculated intravenously with 1 mL of inoculum.
Zinc Gluconate Hydrate (purchased from Energy Chemical, Shanghai, China) and Ribavirin (purchased from Biochempartner, Shanghai, China) was administered orally to each rabbit. Group A (n = 6) 10mg/kg ribavirin; group B (n = 6) 100 mg/kg zinc gluconate; group C (n = 6) 10 mg/kg ribavirin and 100 mg/kg zinc gluconate; group D (n = 6) sterile water. All animals were administered daily with drugs or sterile water and treated until 8 weeks post-inoculation (WPI). The animal study was performed at the Beijing University Health Science Center, in line with previously reported methods (16).
3.3. Zinc Supplementation in Patients with Chronic Hepatitis E
In line with the EASL guidelines, chronic hepatitis E has been defined as viral persistence for over 3 months (3). Non-response to ribavirin has been defined as not reaching virological clearance under ribavirin monotherapy after more than 3 months of treatment. This retrospective case series includes 12 patients from 8 medical centers in Germany and France (Hamburg, Osnabrück, Leipzig, Würzburg, Köln, Hannover, Tübingen, and Paris). Real-time quantitative PCR was performed locally. Zinc supplementation was initiated based on the previously described antiviral effects of zinc (12). The dietary supplement zinc has been administered at dosages of 4 to 36 mg zinc per day, according to the assessment of the attending physicians.
3.4. Statistical Analysis
Data are shown as absolute numbers and percentages or as the median and interquartile range (IQR). Nominal variables were compared using the chi-square test, and metric variables were analyzed using the Mann-Whitney-U-test. Correlation analysis was performed using Spearman’s rho. Statistical analyses were performed using the IBM SPSS software package (version 24).
3.5. Ethical Consideration
This study was conducted according to the guidelines of the Declaration of Helsinki. Data collection at the University Medical Center Hamburg-Eppendorf was approved by the ethics committee of the Medical Council of Hamburg (WF-138/20 and PV7049). Data from participating centers were transmitted anonymously to the University Medical Center Hamburg-Eppendorf and were analyzed in line with the recommendations of the local ethics committees. The Committee of Laboratory Animal Welfare and Ethics, Peking University Health Science Center, approved the animal experiments.
4. Results
4.1. Zinc Inhibits HEV Replication in the Replicon System
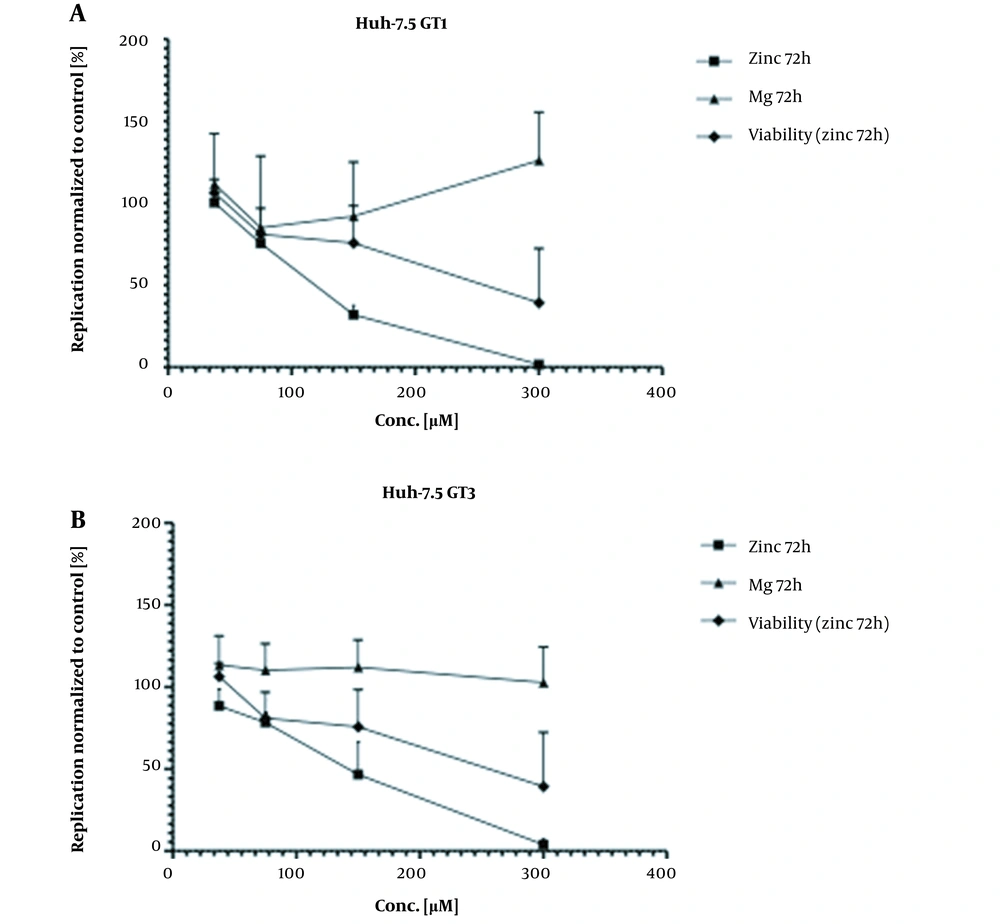
As shown in Figure 1A, our in vitro experiments using a subgenomic replicon system demonstrated that zinc salts inhibited HEV replication in vitro more effectively than magnesium (Mg), which served as a control. For HEV genotypes 3 and 1, the half-maximal inhibitory concentrations (IC50) were 130 µM and 115 µM. The 50% cytotoxicity concentration (CC50) was approximately 300 µM (Figure 1).
4.2. Zinc as a Therapeutic Agent in Experimentally HEV-Infected Rabbits
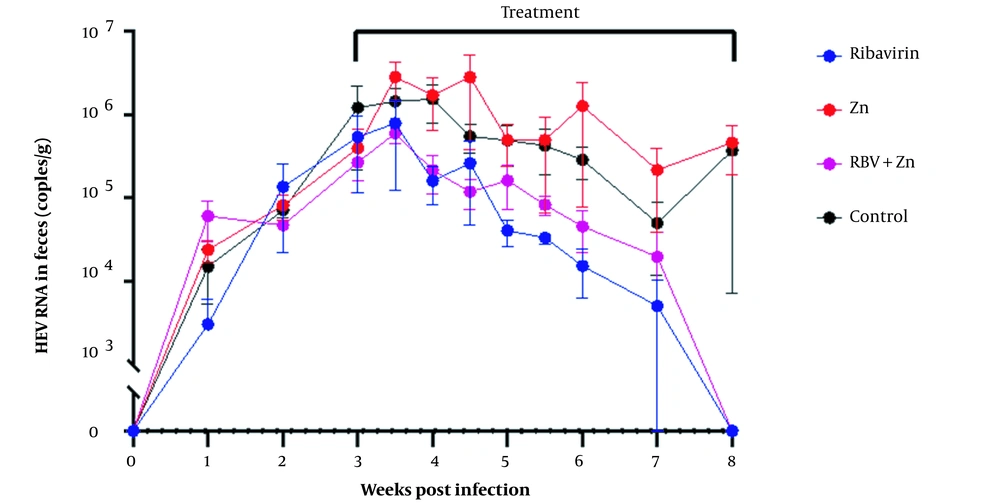
All fecal samples of HEV-infected rabbits tested positive for HEV RNA at 2 WPI, indicating successful infection with HEV-3ra. Treatment was started at 3 WPI since the viral load in fecal samples was high, and the infection was stable. As shown in Figure 2, the treatment lasted for 5 consecutive weeks, and all fecal samples from rabbits treated with ribavirin or ribavirin combined with zinc tested negative for HEV RNA at the end of the treatment. Zinc did not improve the antiviral effect of ribavirin since fecal virus shedding gradually declined in all animals from both groups during treatment and eventually stopped fecal virus shedding at 8 WPI. No significant difference in fecal HEV shedding was found between these 2 groups. Like the control group, virus shedding was continuously observed throughout the experiment in rabbits treated with zinc monotherapy. HEV RNA levels in all fecal samples showed no significant difference between all 4 groups throughout the experiment.
4.3. Zinc as a Supplement in Patients with Chronic Hepatitis E
Twelve patients with chronic hepatitis E without SVR on ribavirin monotherapy received additional zinc supplementation. 4/12 patients (33%) responded to zinc supplementation and achieved SVR, as described below. Characteristics of patients with and without SVR under ribavirin and additional zinc supplementation are shown in Table 1.
| Patients Achieving SVR Under Zinc Supplementation (n = 4) | Patients Without SVR (n = 8) | |
|---|---|---|
| Sex | 3 m (75%) | 3 m (38%) |
| Age, mean (range) | 47 years (35 - 57 years) | 51 years (36 - 70 years) |
| Reason for immunosuppression | -1 HIV; -1 Kidney transplant; -1 Follicular Lymphoma; -1 B-cell Lymphoma with antibody deficiency | -2 Heart transplant; -3 Kidney transplant; -1 Kidney transplant and Plasmocytoma; 1 Kidney + Heart transplant; 1 Lung transplant |
| Ribavirin dosage in mg, mean (range) | 800 (600 - 1000) | 463 (200 - 1000) |
| Zinc dosage in mg (range) | 25 (15 - 36) | 16 (4 - 20) |
| AST | 48 (22 - 83) | 121 (48 - 387) |
| ALT | 167 (74 - 464) | 98 (15 - 255) |
| HEV viral load (begin of riba treatment) | 30.419.566 (4700 - 91.000.000) | 2.538.333 (400.000 - 7.500.000) |
Characteristics of Patients with and Without Achieving Sustained Virological Response Under Ribavirin and Additional Zinc Supplementation a
The median ribavirin dosage was 500 mg daily, and the median zinc dosage was 20 mg daily. The median daily ribavirin dose was higher in those with SVR than those without SVR; however, it did not reach statistical significance (median 800 mg, range 600 - 1000 mg versus median 350 mg, range 200 - 1000 mg; P = 0.15). Furthermore, the administered daily zinc dose was slightly higher in those reaching SVR than in those who did not (median 24.5 mg, range 15 - 36 mg versus median 20mg, range 4 - 20 mg; P = 0.37).
Patient #1, a 47-year-old male HIV-infected patient who developed chronic hepatitis E initially responded to ribavirin (10 mg/kg/d), decreasing viral loads from 210,000 U/mL to 90 U/mL in serum, from 14,000 U/mL to 3,600 U/mL in urine and from 45,000 U/mL to 0 U/mL in the stool within 3 months. Immediately after that, viral loads increased again in all compartments, blood (3,300 U/mL), urine (54,000 U/mL), and stool (120,000 U/mL). Patient #1 received zinc-orotate supplementation of 120 mg/d for 8 weeks in addition to ribavirin therapy, finally achieving SVR after 5 months of total treatment duration.
Patient #2, a 35-year-old renal transplant recipient with an initial viral load of 10,000,000 U/mL, showed a strong decrease in the viral load in the blood under ribavirin (10 mg/kg/d) monotherapy but remained positive in the urine (viral load 2,500,000 U/mL). When adding 50 mg zinc-acetate/d for 4 weeks, a decrease in the viral load in the urine was observed, and the patient remained HEV RNA negative after 21 weeks of total treatment duration.
Patient #3, a 47-year-old female patient with a history of B-cell non-Hodgkin lymphoma, rituximab treatment, and persistent antibody deficiency disorder developing chronic hepatitis E, underwent a 3-month course of ribavirin (1,000 mg/d). Maximum viral loads of 5,500.000 U/mL decreased to 254,000 U/mL. However, the patient did not clear the infection. After a second 4-month course of ribavirin with additional zinc-gluconate of initially 20 mg/d and later 40 mg/d, the patient cured the HEV infection and maintained SVR.
Patient #4, a 57-year-old male patient suffering from follicular lymphoma and history of O-CHOP treatment and chronic hepatitis E, decreased viral load in the serum from initially 90,000,000 U/mL to 1,600 U/mL within 3 months under ribavirin therapy (1,000 mg/d). As the viral load remained stable and did not further decrease, additional zinc supplementation (zinc-orotate 120 mg/d) was given for 24 weeks, and the patient finally cleared the infection. Importantly, we did not observe any overt adverse effects in these patients.
5. Discussion
Up to 10% of patients with chronic hepatitis E experience treatment failure/relapse and do not achieve SVR under ribavirin treatment (6, 7). Thus, novel antiviral strategies are urgently needed for these patients at high risk of developing life-threatening cirrhosis. Zinc salts, widely used as dietary supplements, have been reported to have a broad spectrum of antiviral activity (12, 14). However, the role of zinc supplementation in chronically HEV-infected patients has not yet been studied. Therefore, we aimed to study the antiviral efficacy of zinc against HEV GT3 in vitro in experimentally HEV-infected rabbits and chronically HEV-infected human patients.
Inhibition of HEV genotype 1 RNA-dependent polymerase with zinc salts was reported in vitro in HEV replicons (12). We could reproduce this finding in HEV-GT1 and HEV-GT3 samples in our subgenomic replicon system. However, it has to be noted that with increasing zinc dosage, a simultaneous decrease of cell viability was observed, indicating the potential cytotoxic effects of zinc salts at this dosage.
In a model of acute HEV infection in immunocompetent rabbits with experimental HEV infection, we could not observe a relevant decrease of the viral load in the zinc salt monotherapy group, and based on this pilot project, we could not observe a benefit of zinc in combination with ribavirin in comparison to ribavirin monotherapy (Figure 2).
However, it must be kept in mind that this animal model is not ideal for studying chronic HEV infections, as it is a model of acute self-limiting HEV infection in immunocompetent animals. To observe whether zinc could improve the antiviral effect of ribavirin, a dosage of 10 mg/kg ribavirin, slightly lower than the 20 mg/kg dose which has been reported to inhibit HEV replication in vivo (17), was administered to the rabbits. In summary, based on data from the in vitro replicon system and from the in vivo experimentally infected rabbits, there was no clear visible relevant benefit from zinc supplementation.
However, we also studied 12 patients with chronic hepatitis E without achieving SVR under ribavirin monotherapy. Four (33%) achieved SVR after supplementation with additional zinc. However, most patients (67%) without SVR under ribavirin monotherapy and additional zinc supplementation did not achieve viral clearance, demonstrating that zinc does not seem to be a successful option for all patients. We observed that patients who did not achieve SVR received a slightly lower dose of ribavirin than those who recovered from infection. However, this difference was not statistically significant. Similarly, it appeared that those who achieved SVR received a higher daily dose of zinc, which may indicate a protective effect of zinc. It is important to note that this difference was also not statistically significant.
It can be concluded that most chronically HEV-infected patients do not profit from zinc supplementation. However, individual patients seem to profit from zinc supplementation with ribavirin. It still needs to be determined if immunological, genetic, or hormonal factors might help to differentiate between patients profiting from zinc and those without any benefit.
Based on our small pilot study, we could not detect any relevant differences in gender, age, or underlying disease between patients who responded to zinc and those who did not.
Our study has several limitations. First, the sample size of the patient cohort is rather small. However, this is the first study investigating the antiviral effects of zinc supplementation in a series of chronically HEV-infected individuals. A second limitation is that confounders potentially influencing viral clearance cannot be ruled out due to the study's retrospective design. Therefore, findings should not be overestimated and need to be validated. Prospective data from larger cohorts are needed, preferably from a randomized controlled trial.
Considering our findings and that zinc is an inexpensive and safe dietary supplement, physicians should individually consider whether chronically HEV-infected patients without SVR on ribavirin monotherapy might benefit from additional zinc supplementation. In order to allow a structured recording of such rare cases and to be able to evaluate them in the future, we plan to collect and analyze them in a multicentric manner.
In conclusion, we studied for the first time the role of zinc supplementation in European HEV-GT3 infection in the replicon system and a rabbit model. We could also observe weak antiviral effects in the replicon system and no convincing antiviral effect in experimentally HEV-infected rabbits. However, a potential beneficial effect of zinc salts in combination with ribavirin was observed in one-third of patients with chronic HEV infection without SVR under ribavirin monotherapy.