1. Background
Hepatitis B virus (HBV) infection is a significant life-threatening public health problem, affecting approximately 300 million people worldwide (1). The prevalence of HBV infection varies between 0.1% and 20% in different geographic regions of the world (2). Epidemiological studies in Turkey have shown hepatitis B surface antigen (HbsAg) positivity in approximately 4% of the population (3, 4).
COVID-19 has caused illness and death in millions of people worldwide. During the COVID-19 pandemic, the societal restrictions implemented to stop the spread of infection and decrease patient traffic in hospitals hampered access to health care services for individuals with chronic diseases. Patients diagnosed with chronic hepatitis B (CHB) constitute a significant proportion of these patient groups (5).
With the declaration of COVID-19 as a global pandemic, various precautions were taken in hospitals in Turkey as throughout the world. The follow-up and treatment of non-emergency diseases were largely postponed, and many health care personnel were transferred to units where COVID-19 patients were followed up. The Ministry of Health of the Turkish Republic gave permission for patients who were prescribed drugs for a chronic disease to obtain the drugs from pharmacies by extending report dates without a prescription (6). Therefore, patients being treated and followed up for CHB could not regularly attend the Infectious Diseases Polyclinic. From March 2020 (when the pandemic declaration was made) to February 2022, routine blood and imaging tests could not be made for some CHB patients. It was observed that some patients did not take their drugs regularly or even stopped treatment during this period. Obtaining drugs without a prescription was recently terminated (7).
2. Objectives
This study aimed to provide real-life data showing the effect of the COVID-19 pandemic in terms of the follow-up, treatment, and laboratory test results of patients diagnosed with CHB who could not attend the polyclinic for a long time.
3. Methods
3.1. Study Design and Participants
This prospective, observational study included patients aged > 18 years who presented at 3 centers between 14 February 2022 and 30 March 2022. The subjects had been diagnosed with CHB, receiving antiviral treatment for at least 1 year, and attending regular follow-up appointments before the pandemic.
Written informed consent was obtained from all the study participants.
The patients included in the study were categorized into 3 groups according to their presentation status during the pandemic. Group 1 included patients who had checkups regularly and received treatment during the pandemic. Group 2 included patients who did not have checkups during the pandemic and interrupted or stopped taking the drugs. Group 3 included patients who did not have checkups during the pandemic but continued to take their drugs regularly. The patients in groups 2 and 3 were evaluated after social restrictions due to the COVID-19 pandemic at outpatient clinics.
Patients were excluded from the study if they had not taken their drugs regularly or had polyclinic checkups regularly before the pandemic, had a diagnosis of cirrhosis, were taking HBV prophylaxis because of immunosuppression, had started treatment less than 1 year before the pandemic, or were pregnant.
Patients who had checkups and those who did not were compared regarding demographic data and pre and post-pandemic serological and biochemical parameters, including hepatitis B surface antigen (HbsAg), hepatitis b surface antibody (AntiHbs), hepatitis b e antigen (HbeAg), hepatitis b e antibody (AntiHbe), aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, α-fetoprotein (AFP), calcium, phosphorus, creatinine, glomeruli filtration rate (GFR), cell count parameters, international normalized ratio (INR) and hepatitis B virus (HBV) DNA results.
3.2. Statistical Analysis
Data were analyzed using SPSS version 25.0 (IBM SPSS Inc., Chicago, IL, USA). Descriptive statistics were stated as mean ± SD or median (minimum-maximum) values for continuous variables and as number (n) and percentage (%) for categorical variables. In comparing the groups according to the status of having checkups and continuing drug use, a chi-square test was used for categorical data, and non-parametric Kruskal-Wallis and Mann-Whitney U tests were used for continuous data, as parametric test conditions were not met. To compare the repeated measurements of markers used during checkups before and after the pandemic, Wilcoxon signed-rank and McNemar tests were used for dependent groups. P values less than 0.05 were considered statistically significant.
3.3. Ethical Approval
The necessary permission for the study was obtained from the Clinical Research Ethics Committee of Batman Training and Research Hospital (decision No.: 296, dated: 12/01/2022).
4. Results
A total of 220 patients diagnosed with CHB at Batman Training and Research Hospital, Harran University Medical Faculty Hospital, and Dicle University Medical Faculty Hospital were studied in this research. The subjects consisted of 142 (64.5%) males and 78 (35.5%) females, with a mean age of 44 years (range, 19 - 73 years). Of the patients with CHB known to have been present for a mean of 13 years, 167 (75.9%) had a family history of CHB, and 53 (24%) had a family history of cirrhosis of the liver. The treatments before the pandemic were tenofovir disoproxil fumarate (TDF) in 103 (46.8%) patients, tenofovir alafenamide fumarate (TAF) in 40 patients (18.2%), entecavir (ETV) in 75 (34.1%) patients, and lamivudine in 2 (0.9%) patients. It was learned that 22 (10%) patients were being followed up because of comorbidities.
4.1. Basic Characteristics of the CHB Patients Not Having Checkups During the Pandemic
Of the 220 patients who had received treatment and regularly had checkups before the pandemic, 142 (64.5%) did not go to any follow-up visits during the pandemic. The basic characteristics are shown in Table 1 according to the status of having checkups during the pandemic. No significant difference was determined between patients having or not having checkups during the pandemic regarding the basic characteristics. None of the patients who had checkups had any problems regarding their drugs, and 24 (16.9%) of those who did not have checkups experienced problems regarding their drugs.
| Variables | Had Checkups (n = 78) | Did Not Have Checkups (n = 142) | P Value b |
|---|---|---|---|
| Age (y) | 44.5 (19 - 71) | 44 (19 - 73) | 0.838 |
| Gender | 0.847 | ||
| Male | 51 (65.4) | 91 (64.1) | |
| Female | 27 (34.6) | 51 (35.9) | |
| Education level | 0.376 | ||
| No formal education | 13 (16.7) | 27 (19.0) | |
| Primary school | 25 (32.1) | 56 (39.4) | |
| High school or university | 40 (51.3) | 59 (41.5) | |
| Place of residence | 0.605 | ||
| Village | 9 (11.5) | 22 (15.5) | |
| Town | 12 (15.4) | 17 (12.0) | |
| City | 57 (73.1) | 103 (72.5) | |
| Comorbidity present | 20 (25.6) | 40 (28.2) | 0.687 |
| Use of other drugs | 33 (42.3) | 60 (42.3) | 0.994 |
| Time since diagnosis of hepatitis B (y) | 14 (3 - 30) | 12 (3 - 30) | 0.555 |
| Duration of taking hepatitis B treatment (y) | 6 (3 - 20) | 6 (3 - 20) | 0.641 |
| Family history of hepatitis B | 57 (73.1) | 110 (77.5) | 0.467 |
| Family history of cirrhosis | 16 (20.5) | 37 (26.1) | 0.358 |
| Treatment received | 0.080 | ||
| ETV | 22 (28.2) | 53 (37.3) | |
| TDF | 34 (43.6) | 69 (48.6) | |
| TAF | 21 (26.9) | 19 (13.4) | |
| Lamivudine | 1 (1.3) | 1 (0.7) | |
| Problems in obtaining the drug | - | 24 (16.9) | NE |
| COVID-19 vaccination | 68 (87.2) | 123 (86.6) | 0.907 |
| COVID-19 infection | 28 (35.9) | 64 (45.1) | 0.187 |
Abbreviations: ETV, entecavir; TDF, tenofovir disoproxil fumarate; TAF, tenofovir alafenamide fumarate; NE, not evaluated.
a Values are expressed as No. (%) or median (minimum - maximum).
b Chi-square test, Mann-Whitney U test.
4.2. Reasons for CHB Patients Not Having Checkups During the Pandemic
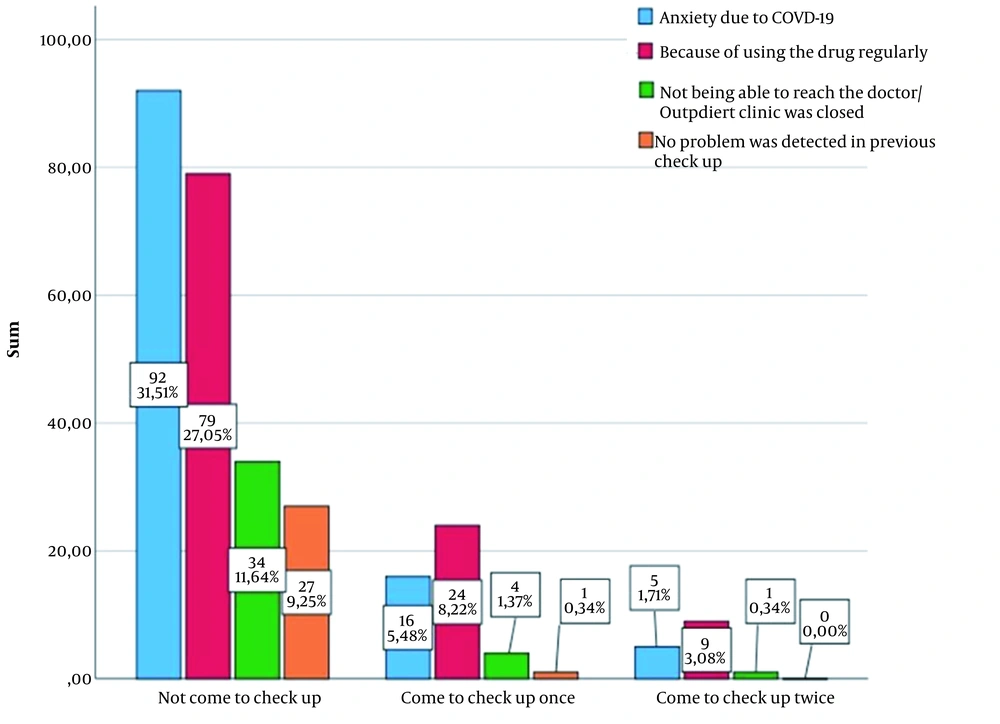
It was determined that 39 (17.7%) patients continued to regularly have checkups during the COVID-19 pandemic, 39 (17.7%) had irregularly (once or twice during the 2-year period of the pandemic), and 142 (64.5%) did not have any checkups. The reasons for not having checkups and having irregular checkups are shown in Figure 1. At least 1 reason was stated by 181 patients, 2 by 61 patients, and 3 by 25 patients. The most common reason for not having checkups was concern about COVID-19 infection. Those who had 1 or 2 checkups stated most often that they did not feel the need to continue taking the drugs.
4.3. Basic Characteristics of CHB Patients According to the Status of Continuing Treatment During the Pandemic
Of the patients receiving treatment because of CHB, 34 (15.5%) stopped treatment during the pandemic. These patients were determined to have remained without treatment for a mean of 9.18 ± 7.55 months. The basic characteristics of CHB patients according to the status of continuing treatment during the pandemic are shown in Table 2. The patients who stopped treatment were determined to have a younger mean age, and the majority were female (P = 0.001 and P = 0.043, respectively). The patients who stopped treatment were seen to have been receiving antiviral treatment for a shorter period than those who continued treatment (P = 0.013). It was observed that most patients with a family history of liver cirrhosis stopped treatment (P = 0.036). A significantly high rate of patients who experienced problems with their drugs stopped treatment (P < 0.001). A higher rate of patients who had had a COVID-19 vaccination was in the group that continued treatment (P < 0.001).
| Variables | CHB Treatment | P Value b | |
|---|---|---|---|
| Continued Treatment (n = 186) | Stopped Treatment (n = 34) | ||
| Age (y) | 45 (19 - 73) | 36.5 (23 - 59) | 0.001 |
| Gender | 0.043 | ||
| Male | 125 (67.2) | 17 (50.0) | |
| Female | 61 (32.8) | 17 (50.0) | |
| Education level | 0.592 | ||
| No formal education | 35 (18.7) | 5 (14.7) | |
| Primary school | 70 (37.4) | 11 (32.4) | |
| High school or university | 81 (43.5) | 18 (52.9) | |
| Place of residence | 0.624 | ||
| Village | 25 (13.4) | 6 (11.5) | |
| Town | 26 (14.0) | 3 (15.4) | |
| City | 135 (72.6) | 25 (73.5) | |
| Comorbidity present | 54 (29.0) | 6 (17.6) | 0.170 |
| Use of other drugs | 82 (44.1) | 11 (32.4) | 0.203 |
| Time since diagnosis of hepatitis B (y) | 12 (3 - 30) | 12 (4 - 30) | 0.911 |
| Duration of taking hepatitis B treatment (y) | 7 (3 - 20) | 5 (3 - 19) | 0.013 |
| Family history of hepatitis B | 138 (74.2) | 29 (85.3) | 0.164 |
| Family history of cirrhosis | 40 (21.5) | 13 (38.2) | 0.036 |
| Treatment received | 0.667 | ||
| ETV | 62 (33.3) | 13 (38.2) | |
| TDF | 86 (46.2) | 17 (50.0) | |
| TAF | 36 (19.4) | 4 (11.8) | |
| Lamivudine | 2 (1.1) | - | |
| Problems in obtaining the drug | 10 (5.4) | 14 (41.2) | < 0.001 |
| COVID-19 vaccination | 168 (90.3) | 23 (67.6) | < 0.001 |
| COVID-19 infection | 81 (43.5) | 11 (32.4) | 0.224 |
Abbreviations: ETV, entecavir; TDF, tenofovir disoproxil fumarate; TAF, tenofovir alafenamide fumarate.
a Values are expressed as No. (%) or median (minimum - maximum).
b Chi-square test, Mann-Whitney U test.
4.4. Changes in the Markers Used in the Follow-up of CHB Before and After the Pandemic According to the Status of Continuing Treatment
Before the pandemic, 219 (99.5%) patients were HBsAg positive. HBsAg loss occurred in 1 patient who stopped treatment and in 2 patients who continued treatment during the pandemic. In the 2 patients who continued treatment, anti-HBs seroconversion developed. Before the pandemic, HBeAg was determined in 18 (8.2%) patients and anti-HBe positivity in 197 (89.5%) patients. Both were positive in 1 patient and negative in 6 patients. HBeAg loss occurred in 1 patient who stopped treatment and in 2 patients who continued treatment during the pandemic, and anti-HBe seroconversion was observed in these 3 patients.
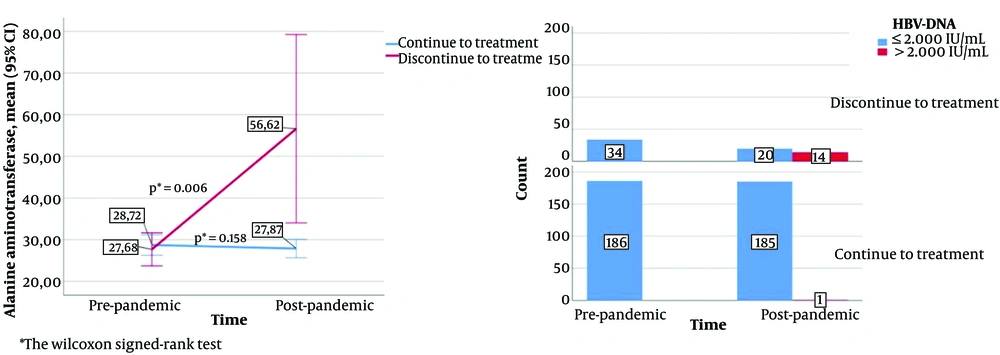
The changes in HBV-DNA and ALT values according to the status of continuing treatment during the pandemic are shown in Figure 2. A statistically significant increase was observed in the ALT value of those who stopped treatment compared to the pre-pandemic value (P = 0.006), and no significant change was observed in those who continued treatment (P = 0.158). Following the pandemic, HBV DNA > 2000 IU/mL was determined in 14 (41.1%) patients who stopped treatment and in 1 (0.5%) patient who continued treatment. As the HBV-DNA value was negative in all the patients before the pandemic, no statistical result could be obtained from the changes.
The changes in some biomarkers used in the follow-up of CHB according to the status of continuing treatment during the pandemic are shown in Table 3. AST values after the pandemic were determined to be statistically significantly low in the patients who continued treatment and high (but not at a statistically significant level) in those who stopped treatment (P = 0.002 and Pc = 0.081, respectively). The serum calcium level was significantly reduced after the pandemic in the patients who stopped treatment (P = 0.048). When the effect of stopping treatment was evaluated, this decrease was associated with having stopped the use of TDF (the mean calcium value of those who stopped TDF was 9.58 ± 0.31 before the pandemic and 9.40 ± 0.34 after the pandemic, P = 0.049).
| Variable s and Treatment | Pre-pandemic | Post-pandemic | P Value a |
|---|---|---|---|
| ALT | |||
| Continuing | 28.72 ± 17.02 | 27.87 ± 15.20 | 0.158 |
| Terminated | 27.68 ± 11.36 | 56.62 ± 64.78 | 0.006 |
| AST | |||
| Continuing | 27.35 ± 9.73 | 25.71 ± 7.78 | 0.002 |
| Terminated | 26.33 ± 9.25 | 34.45 ± 21.71 | 0.081 |
| AFP | |||
| Continuing | 2.94 ± 2.74 | 3.11 ± 2.40 | 0.085 |
| Terminated | 2.67 ± 2.31 | 3.20 ± 3.04 | 0.050 |
| INR | |||
| Continuing | 1.07 ± 0.13 | 1.06 ± 0.11 | 0.542 |
| Terminated | 1.08 ± 0.12 | 1.11 ± 0.10 | 0.098 |
| Thrombocyte count | |||
| Continuing | 236 ± 60 | 241 ± 63 | 0.098 |
| Terminated | 231 ± 59 | 235 ± 64 | 0.388 |
| Creatinine | |||
| Continuing | 0.80 ± 0.22 | 0.82 ± 0.36 | 0.340 |
| Terminated | 0.73 ± 0.19 | 0.70 ± 0.18 | 0.346 |
| Phosphorus | |||
| Continuing | 3.06 ± 0.54 | 3.04 ± 0,52 | 0.746 |
| Terminated | 3.14 ± 0.39 | 3.04 ± 0.51 | 0.161 |
| Calcium | |||
| Continuing | 9.40 ± 0.64 | 9.43 ± 0.61 | 0.605 |
| Terminated | 9.54 ± 0.61 | 9.40 ± 0.35 | 0.048 |
| Albumin | |||
| Continuing | 41.21 ± 4.83 | 41.14 ± 4.93 | 0.528 |
| Terminated | 40.87 ± 4.90 | 40.21 ± 4.27 | 0.074 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio; AFP, α-fetoprotein.
a Wilcoxon signed-rank test.
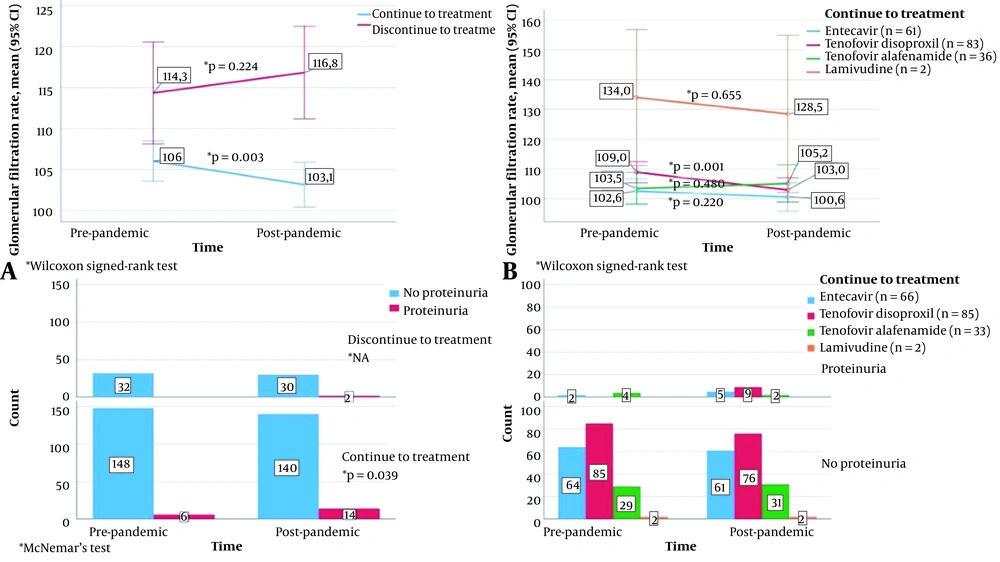
The relationship between the status of continuing treatment during the pandemic and the change in GFR and proteinuria is shown in Figure 3. The GFR value was significantly reduced after the pandemic in those who continued treatment (P = 0.003). This decrease was particularly associated with continuing TDF treatment; in these patients, the mean GFR value was significantly reduced (P = 0.001). However, the mean GFR value increased in those who stopped treatment, which was not statistically significant (P = 0.224). A total of 186 patients were evaluated regarding proteinuria before and after the pandemic. In patients who continued treatment, proteinuria was observed in 6 (3.8%) patients before the pandemic and in 14 (9.1%) patients after the pandemic, which was statistically significant (P = 0.039). After the pandemic, proteinuria was observed in 2 patients who stopped treatment, but as proteinuria was not observed in this group before the pandemic, a statistical result could not be obtained. In 9 patients without proteinuria before the pandemic and those who continued to use TDF, proteinuria was observed after the pandemic.
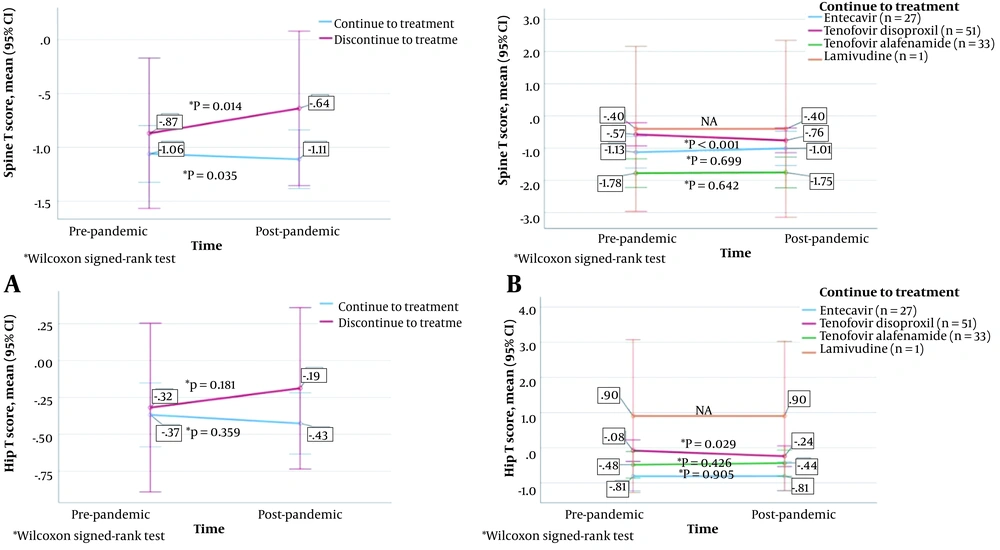
The changes in bone mineral density (BMD) according to the status of continuing treatment during the pandemic (as well as according to the treatment taken by those continuing) are shown in Figure 4. The vertebra T score was found to be significantly higher after the pandemic than before the pandemic in the patients who stopped treatment (P = 0.014), as well as to be significantly lower in those who continued treatment (P = 0.035). The femur T score decreased after the pandemic compared to the pre-pandemic scores in both patients who stopped and continued treatment, but the differences were not statistically significant (P = 0.181 and P = 0.359, respectively). Both the vertebra T score and femur T score were significantly lower after the pandemic than before the pandemic in the patients who continued TDF treatment (P < 0.001 and P = 0.029, respectively).
4.5. Changes in Treatment After the Pandemic
After the pandemic, the treatment of 156 (83.4%) patients was not changed, the treatment of 11 (5.9%) patients was changed to ETV because of the development of osteopenia/osteoporosis, and the treatment of 18 (9.6%) patients was changed to TAF because of the development of osteopenia/osteoporosis or renal side effects. To switch to a more potent drug, the treatment in 1 (0.5%) patient was changed from lamivudine to TDF. Of the patients who stopped treatment, it was re-started with the same drug in 27 (79.5%) patients. Treatment was not re-started at the request of 6 (17.6%) patients, and it was changed to TAF in 1 (2.9%) patient because of the development of osteoporosis.
5. Discussion
In the long-term treatment management of all chronic diseases, maintaining the efficacy of the treatment and monitoring side effects are critical. Therefore, CHB patients receiving treatment should undergo liver function tests once every 3 - 4 months in the first year and every 6 months thereafter. The serum HBV-DNA level should be examined once every 3 - 4 months in the first year and at 6- to 12-month intervals thereafter. When HBV DNA is not determined, the HBsAg and anti-HBs levels should be examined once every 12 months (8). The CHB patients being followed up at 3-month intervals before the pandemic could not be seen for polyclinic checkups because of the COVID-19 pandemic restrictions. The right to be able to obtain the drugs without a prescription laid the foundation for these patients not to see the need to present at the polyclinic. This study showed that a negative effect should be considered on chronic diseases such as hepatitis b infection because of the extension of control visits during pandemics. The aim of this study was to evaluate the clinical, radiological (bone mineral densitometer), serological, biochemical, and molecular results of the patients who did not have polyclinic checkups.
Of the 220 patients who had checkups regularly before the pandemic, 142 (64.5%) had no checkup for approximately 2 years. Only 39 (17.7%) patients had regular checkups. Of the reasons given by the patients for not having checkups, the main reason was concern about COVID-19 infection. Another study reported that pregnant women did not have regular routine checkups during the pandemic as they felt uncomfortable (9). In a study in Wuhan (China), it was seen that 41.9% of pregnant women refused to go to any hospital because of the fear of infection and would postpone antenatal care and admission to the hospital before birth (10). Obstetricians in India reported that the most common concern of pregnant women was that they would contract the infection during hospital visits for antenatal checkups and ultrasounds (11). It can be predicted that this situation would be seen more in CHB patients because most of these patients have no complaints. A previous study reported that one of the main reasons for not having a viral hepatitis test and accessing treatment was the fear of going to a health care facility because of COVID-19 (12).
In the current study, 34 patients (who were young and mostly female) stopped treatment during the pandemic. In the patients who had been receiving antiviral treatment for a longer time, the rate of continuing to take the drugs was higher. It was thought that the importance of the disease could be better understood by those having checkups for a longer period. An interesting finding was that a significantly higher rate of patients with a family history of cirrhosis of the liver stopped treatment. The compliance to the treatment might be low because they may have believed that cirrhosis is the final inevitable outcome of CHB. It was observed that most patients who continued treatment had a COVID-19 vaccination, which was statistically significant. Due to the protection of the vaccination, it can be considered that the patients lost their concerns about COVID-19 and continued the treatment of CHB. These patients might have compliance with both their treatments and COVID-19 vaccinations. In the later stages of the pandemic (when there was an increase in COVID-19 vaccinations), a study conducted in Canada showed that with this effect, there was an increase in hospital presentations (13).
HBsAg seroclearance and its conversion to an antibody against hepatitis B surface antigen (anti-HBs) is uncommon in chronic infections. In an Asian population, the annual incidence of HBsAg clearance was 0.33% - 5.0% with antiviral treatments and 0.5% - 2.66% without antiviral treatments (14-17). In a study in Turkey, HBsAg seroclearance was developed in only 2 of 173 HBeAg-negative patients, and anti-HBs seroconversion was not determined (18). In the current study, HBsAg loss was seen in 1 patient who stopped treatment and in 2 patients who continued treatment, and anti-HBs seroconversion was developed in 2 patients who continued treatment. Similarly, HBeAg loss was observed in 1 patient who stopped treatment and in 2 patients who continued treatment, and anti-HBe seroconversion was developed in 2 patients who continued treatment. Seroconversion was developed more in patients who continued treatment, which is consistent with previous studies. However, the timing of seroconversion developed in patients who did not regularly have checkups could not be determined. It is recommended that treatment be continued for 1 more year after seroconversion (8). As this timing was not clearly known, patients will have to remain in treatment for a longer period.
There are 2 formulations of tenofovir for the treatment of HBV: TDF and TAF. ETV is generally well tolerated in CHB. In patients with a high potential for side effects related to the bones and kidneys, it is recommended that treatment be changed from TDF to TAF or ETV (8, 19, 20). Previous studies have reported that long-term use of TDF results in a decrease in BMD and an increase in renal toxicity (20-22). Patients who changed from TDF to TAF showed a significant improvement in estimated glomerular filtration rate (eGFR) levels and hip and spine T scores (20). In another study, it was observed that after 2 years of TDF treatment, eGFR significantly decreased. In patients who received ETV, eGFR remained stable throughout 3 years and significantly improved after 4 and 5 years (23). Consistent with previous studies, a decrease in eGFR was observed in patients who continued TDF treatment. Moreover, in 9 patients who continued to use TDF and had not been determined by proteinuria before the pandemic, the treatment was changed to ETV or TAF after the pandemic as proteinuria was determined. Previous studies have shown that proteinuria is one of the side effects seen in TDF regimens (24, 25). When proteinuria develops in HBV patients using TDF, it is recommended that the treatment be changed to TAF or ETV (6, 7, 26).
In the current study, the vertebra T score was found to be significantly higher after the pandemic than before the pandemic in patients who stopped treatment, as well as to be significantly lower in those who continued treatment. In addition to not being the only significant factor for the reduction in BMD, drugs seems to have a large effect on it. Both the vertebra T score and the femur T score were significantly lower after the pandemic than before the pandemic in the patients who continued TDF treatment. These results are consistent with previous studies (20).
Of the 34 (15.5%) patients who stopped taking antiviral treatment during the pandemic, 20 (58.9%) were determined by HBV DNA < 2000 IU/mL and 14 (41.1%) with HBV DNA > 2000 IU/mL. Of those with HBV DNA < 2000 IU/mL, 6 did not wish to resume treatment, and in 2 of these patients, the result was negative. Acceptable treatment termination is stated in the guidelines as continuous virological improvement [HBV DNA levels were not determined by sensitive polymerase chain reaction (PCR)] (5, 9). In a previous study of 504 HBeAg-negative, non-cirrhotic patients who were followed up without treatment for at least 30 months after previous ETV or TDF, virological recurrence was determined in 81 patients. Of these 81 patients, HBV DNA > 2000 IU/mL was determined in 52 patients, and following virological recurrence in 29 patients, a permanent virological dominance (HBV DNA < 2000 IU/mL) was seen at least 1.5 years later (27). In another study, of the 250 patients for whom ETV was stopped, there was permanent virological dominance in 71 (28.4%) patients and virological recurrence in 35 (15%) patients. In patients who developed virological recurrence, clinical recurrence did not develop, or there was no need for treatment again (28). Virological recurrence was determined at a higher rate in the current study than in the literature. In addition, a significant increase in the ALT value was determined in patients who stopped treatment. All guidelines state that normalization of ALT is an important factor in the follow-up of CHB and is a marker of biochemical response (7, 25, 29). Such a result seen in patients who stopped treatment is supported by the guidelines.
5.1. Conclusion
The COVID-19 pandemic had negative effects on the follow-up of patients with chronic diseases. The most common reason for not having checkups was concerns about COVID-19 infection. The primary negative effects encountered in the follow-up of patients were biochemical impairment and virological recurrence developed as a result of stopping treatment. Following the use of TDF, a decrease was seen in BMD, a fall in the level of eGFR, and side effects such as proteinuria. Both HBsAg seroconversion and HBeAg seroconversion were observed more often in patients who continued treatment. To continue antiviral treatments and follow up side effects, patients should have regular checkups.