1. Background
Nonalcoholic fatty liver disease (NAFLD) is the commonest chronic liver disease, in which the accumulation of large triglyceride droplets is observed in hepatocytes, causing over 5% of liver weight without chronic alcohol use (1-3). It causes several diseases, such as steatohepatitis, simple steatosis, cirrhosis, and sometimes hepatocellular carcinoma. The NAFLD prevalence is 20 to 30% in the general population , raising to 70 - 90% in diabetic and obese patients (3).
The NAFLD pathogenesis is not clear, however, we can explain it by the multi hypothesis: The first hit is steatosis (due to insulin resistance), the second one is inflammation and oxidative stress (leading to disease progression) (4, 5), and the third one is impairment of hepatocyte proliferation progenitors (5).
The epidemiology of type 2 diabetes and obesity is increasing, thus, NAFLD is now a public health issue, and exhibit a prevalence of 6 - 35% in adult people in the world (6). Adherence to lifestyle and dietary changes can be regarded as the first step in the correction of hepatic fat accumulation and prevention of NAFLD progression to nonalcoholic steatohepatitis (NASH). Nonetheless, many patients cannot have proper weight control, with low compliance with lifestyle changes. Despite the high NAFLD prevalence, finding successful and safe treatments for NAFLD is widely considered. Therefore, medicinal plants and natural products have been considered because of their availability, cost-effectiveness, multi-target effect, and safety (7-9).
Grape (Vitis vinifera) is a widely consumed fruit worldwide. Grape seeds have high levels of antioxidants, such as phenolic compounds (mainly tannins), which can decrease the risk of chronic disease through protection against free radical-associated damage. Grape seeds are a rich source of epicatechin, catechin, and epicatechin-3-O-gallate called proanthocyanidins (PAs). PAs in grape seeds have anti-inflammatory, anti-allergic and anti-arthritis, properties, scavenge free oxygen radicals, inhibit skin aging, and suppress UV radiation-related peroxidation activity (10).
2. Objectives
We assessed the effects of grape seed extract (GSE) on nonalcoholic fatty liver. We hypothesize that treatment with GSE will ameliorate fatty liver and improve liver functions, lipid profiles, and fasting blood sugar (FBS) in nonalcoholic fatty liver patients.
3. Methods
3.1. Study Design
The current randomized, controlled clinical trial was done between January 2021 and March 2022 on patients referring to the specialized and subspecialized clinics affiliated with the Abadan University of Medical Sciences. Ultrasonography was done by single sonographers to determine fatty liver. The fatty liver degree was categorized as mild and moderate.
3.2. Inclusion and Exclusion Criteria
NAFLD patients were evaluated according to the inclusion criteria included 20 - 50 years of age and body mass index (BMI) of 30 - 40 kg/m2. Exclusion criteria included alcohol use, lactation or pregnancy, being athlete and menopausal, inflammatory conditions, like hypertension, infection, family history of hyperlipidemia, cardiovascular disease, renal, lung, or liver disease, biliary disease, cancer, liver transplantation, known autoimmune disease, injuries and burns during the research, surgery in the last three months, taking medications, like insulin sensitivity enhancers, antihypertensive, statins, hepatotoxic drugs, estrogens, contraceptive pills and, and antioxidant supplementation in the last two months.
3.3. Assignment Randomization and Blinding
A sample size of 45 was determined for each group using the following formula and considering σ1 = 41.76, σ2 = 39.56, μ1 = 198.59, μ2 = 174.38, α = 0.05 and power of %80 (β = 0.20)(11). The assignment of individuals to groups was performed random allocation (Simple randomization) using random allocation software. To blind the research, numbered envelopes were prepared, and the name of the group to which the individuals belonged, the letters “A” and “B,” were put into each envelope. As each participant entered the study, the envelopes were opened sequentially, and the participant entered the assigned group. Participants and individuals who collected the information were not aware of the assignment of individuals to the groups; only the researcher was aware of it. The comparison group received a placebo.
Grapex and placebo powder were filled into capsules matched by shape, size, and color and dispensed in similar blinded bottles.
3.4. Intervention
Dried hydro-alcoholic GSE (Vitis vinifera) was prepared by Soha JIsa Company (Tonekabon, Iran). The extract’s total anthocyanin content (TAC) was evaluated through the pH-differential method (12).
TAC extract content was calculated to be 190 mg/g of dried extract, equivalent to 38 mg in each capsule. Formulation of the drug into the capsule dosage form (200 mg) was performed at Pharmaceutical Incubation Center, Ahwaz, Iran. The allocated name of such capsules was Grapex.
Eligible subjects were randomly assigned to Grapex (GSE, 200 mg/d; n = 45) or placebo (n = 45). The patients received GSE or placebo for 2 months.
3.5. Main Outcomes
3.5.1. Anthropometric Measurements
The subjects’ weight and height were evaluated considering an accuracy of 0.1 kg and 0.1 cm , respectively. Measurements were done with minimal clothing while standing, without shoes at baseline and eighth weeks. BMI was computed as weight in kg divided by height in m squared.
3.5.2. Blood Sampling and Biochemical Assessments
The cubital vein was used to collect fasted blood samples at the beginning and after four and eight weeks of treatment. Blood specimens were centrifuged at 2000 – 2500 g for 10 minutes to isolate the serum. Serum specimens were stored at -80°C until assessments. High-density lipoprotein (HDL), Total cholesterol, low-density lipoprotein (LDL), triglycerides (TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and FBS were measured at baseline, 1 month after consumption and at the end of research (2 months) by routine enzymatic assays using Pars Azmoon commercial kits (Iran).
3.6. Ethical Consideration
The research protocol was approved by the Ethics Committee of Abadan University of Medical Sciences (IR.ABADANUMS.REC.1397.008) and registered at the Iranian Registry of Clinical Trials (IRCT20200921048783N2). Written informed consent was taken from all participants.
3.7. Statistical Analysis
Statistical analysis was done by SPSS 18. The normality of the data was checked using the Kolmogorov-Smirnov test. The difference between groups in terms of age, gender, severity of disease, weight, and BMI variables was assessed using the chi-square test and independent samples t-test. The changes in weight and BMI in each group at baseline and the end of the study were assessed using a paired-samples t-test. The differences between groups in investigating parameters (AST, ALT, TG, Col, LDL, HDL, FBS) at any time were assessed using the Mann-Whitney U test. Changes of parameters in each group at different times were analyzed using the Friedman test, and the Wilcoxon post-hoc test was used for pairwise comparison of parameters in each group. Graph productions were carried out using GraphPad Prism 5 (USA).
4. Results
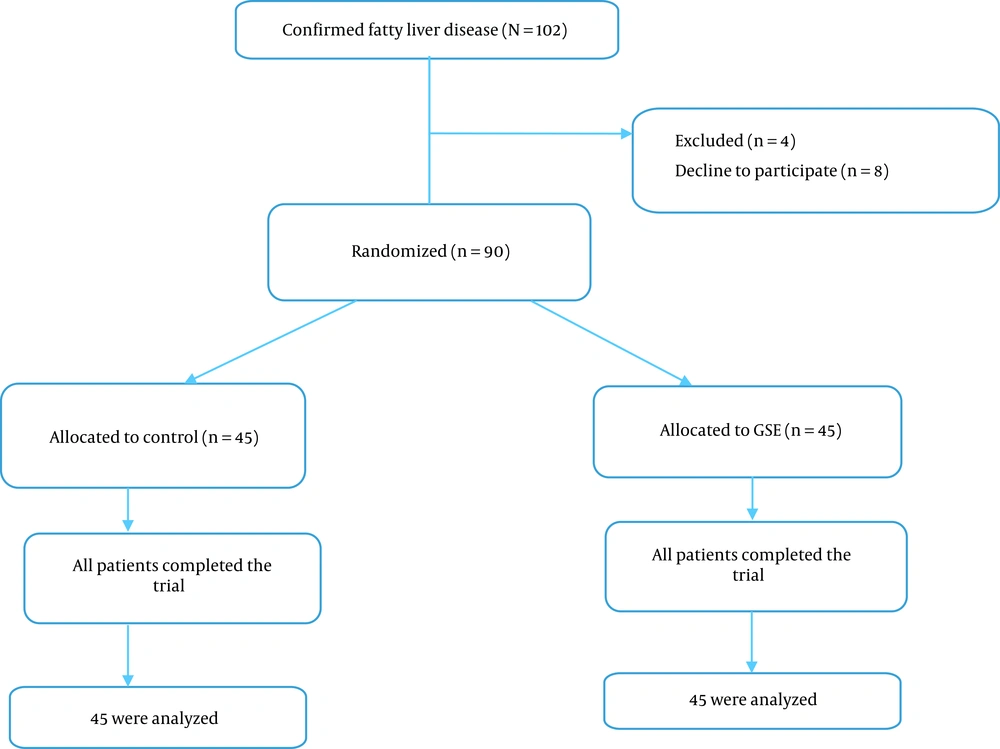
A total of 102 patients with confirmed fatty liver were assessed for eligibility from January 2021 to March 2022. Twelve cases were excluded for the reasons described in Figure 1. The remaining 90 cases were assigned randomly to GSE (n = 45) and control groups (n = 45). All patients in the control and GSE groups (n = 90) completed the trial and were included in the statistical analysis (Figure 1). Nobody reported any side effects of GSE usage, and it was well tolerated at 200 mg twice a day.
In this study, 26 women and 19 men were examined in each group. The participants in the 2 groups showed no difference in weight, BMI (at the beginning and end of the study), gender, age, and the severity of the disease (Table 1). The mean weight and BMI at the beginning and the end of the study in the control group showed significant differences, but no significant difference was observed in the intervention group.
| Variables | Control Group | Intervention Group | P-Value |
|---|---|---|---|
| Gender | 1 | ||
| Male | 26 (57.8) | 26 (57.8) | |
| Female | 19 (42.2) | 19 (42.2) | |
| Severity of disease | 0.67 | ||
| Mild | 24 (53.3) | 22 (48.9) | |
| Moderate | 21 (46.7) | 23 (51.1) | |
| Age | 36.04 ± 9.40 | 37.71 ± 9.39 | 0.40 |
| Weight 1 | 78.38 ± 8.48 | 77.87 ± 7.61 | 0.76 |
| Weight 2 | 78.56 ± 8.54 | 77.69 ± 7.49 | 0.61 |
| P-value | 0.04 | 0.07 | - |
| BMI 1 | 28.36 ± 1.75 | 28.08 ± 2.50 | 0.54 |
| BMI 2 | 28.42 ± 1.76 | 28.01 ± 2.45 | 0.36 |
| P-value | 0.04 | 0.07 | - |
Abbreviation: BMI, body mass index.
a Values are expressed as mean ± SD or No. (%).
Table 2 shows the median and interquartile range of the examined parameters in the intervention and control groups. All the investigated factors showed no significantly difference between the control and intervention groups at baseline, however, in the first and second month, there was a significant difference between them, except for HDL and FBS. The amount of HDL had a significant difference between the 2 groups only in the first month, and there was no significant difference at other times. The groups showed no significant difference in FBS in all investigated times.
| Parameter | Control Group Median (P25-P75) | Intervention Group Median (P25-P75) | P-Value a |
|---|---|---|---|
| AST | |||
| Baseline | 55 (45.5 - 65.5) | 52 (46.5 - 68) | 0.72 |
| First month | 57 (45.5 - 67) | 44 (40 - 60) | 0.004 |
| Second month | 58 (46.5 - 68) | 40 (38 - 51) | 0.000 |
| P-value b | 0.000 | 0.000 | - |
| ALT | |||
| Baseline | 53 (47 - 60.5) | 53 (50 - 59) | 0.37 |
| First month | 55 (50 - 62) | 48 (46 - 51) | 0.001 |
| Second month | 56 (51 - 62) | 41 (39.5 - 43.5) | 0.000 |
| P-value b | 0.001 | 0.000 | - |
| TG | |||
| Baseline | 247 (215 - 277.5) | 231 (215.5 - 279) | 0.45 |
| First month | 251 (220 - 280) | 220 (200 - 250.5) | 0.006 |
| Second month | 257 (218 - 281) | 201 (188.5 - 222.5) | 0.000 |
| P-value b | 0.80 | 0.000 | - |
| Cholesterol | |||
| Baseline | 188 (180 - 210) | 187 (173 - 214) | 0.52 |
| First month | 190 (183 - 214) | 173 (168 - 200) | 0.000 |
| Second month | 190 (183 - 210) | 158 (153 - 180.5) | 0.000 |
| P-value b | 0.26 | 0.000 | - |
| LDL | |||
| Baseline | 132 (118.5 - 141) | 125 (120 - 137.5) | 0.49 |
| First month | 130 (117 - 141) | 120 (116.5 - 130) | 0.006 |
| Second month | 134 (118.5 - 141.5) | 112 (109 - 119) | 0.000 |
| P-value b | 0.75 | 0.000 | - |
| HDL | |||
| Baseline | 85 (77 - 87) | 87 (78 - 88) | 0.06 |
| First month | 87 (77.5 - 88) | 88 (83 - 88) | 0.03 |
| Second month | 87 (78 - 88) | 87 (78.5 - 88) | 0.60 |
| P-value b | 0.17 | 0.02 | - |
| LDL/HDL | |||
| Baseline | 1.57 (1.38 - 1.86) | 1.51 (1.41 - 1.50) | 0.28 |
| First month | 1.57 (1.40 - 1.80) | 1.37 (1.34 - 1.50) | 0.000 |
| Second month | 1.56 (1.42 - 1.80) | 1.34 (1.26 - 1.47) | 0.000 |
| P-value b | 0.43 | 0.000 | - |
| FBS | |||
| Baseline | 79 (73 - 82.5) | 78 (76 - 81) | 0.88 |
| First month | 78 (73 - 82.5) | 79 (76 - 81) | 0.71 |
| Second month | 77 (73 - 82) | 78 (76 - 81) | 0.93 |
| P-value b | 0.67 | 0.03 | - |
Abbreviations: AST, aspartate aminotransferase; HDL, high-density lipoprotein; TG, triglyceride; ALT, alanine aminotransferase; LDL, low-density lipoprotein; FBS, fasting blood sugar.
a P-value obtained from Mann-Whitney U test
b P-value calculated by Friedman test
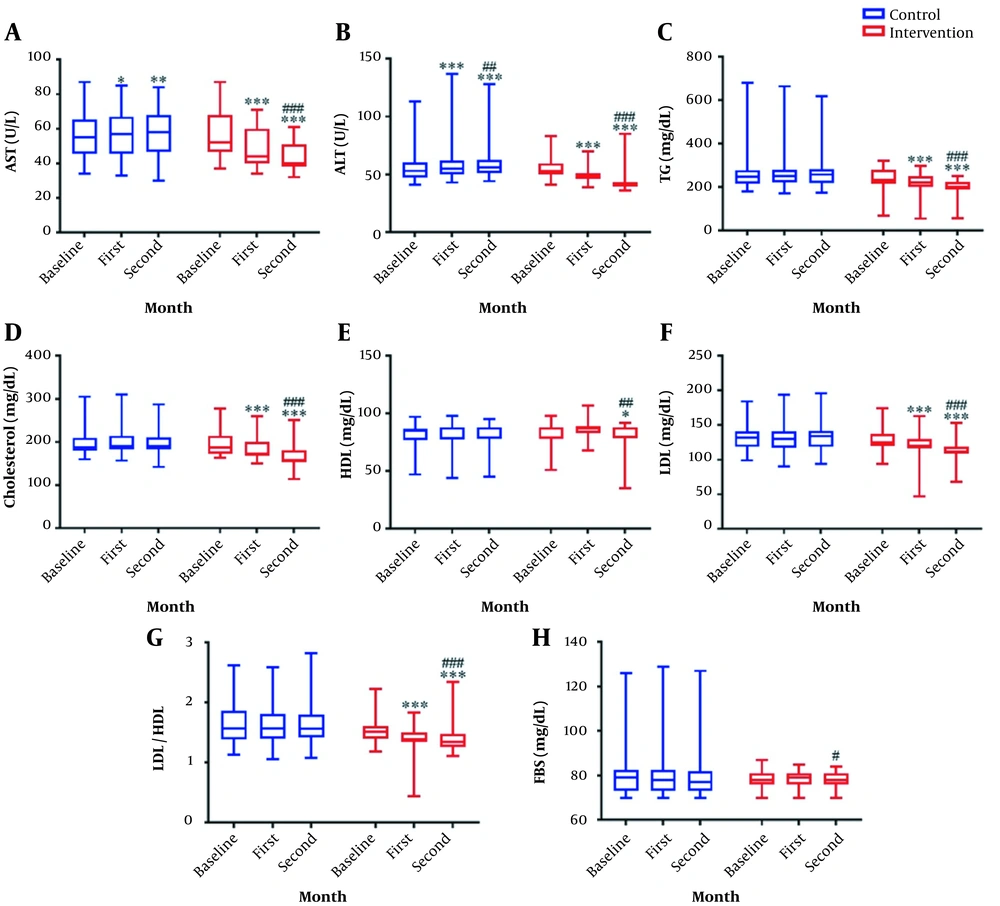
The Friedman test results indicated that the amount of AST and ALT increased over time in the control group and reduced in the intervention group; such alterations were significant. No significant difference was observed in the amount of TG, cholesterol, LDL, HDL, LDL/HDL, and FBS in the control group over time, while there were significant alterations in the intervention group.
According to the results of the Wilcoxon post-hoc test and Figure 2, the amount of AST in the control group was significantly different between baseline and first month (P = 0.01); also, there was a significant difference between baseline and second month (P = 0.004), nonetheless, no significant difference was found between the first and second month (P = 0.13). In the intervention group, the pairwise comparison of all times was significant (P < 0.001).
Comparison of parameter changes within and between groups over time. Grapex (200 mg/d of Grape seed extract) was used in the intervention group (n = 45) and a placebo in the control group (n = 45). The intervention was continued for 8 weeks. The parameters were checked on the first day and the end of the first and second month in both groups. (A) The graph represents the level of aspartate aminotransferase. (B) The graph represents the level of alanine transaminase. (C) The graph represents the level of triglyceride. (D) The graph represents the level of cholesterol. (E) The graph represents the level of high-density lipoprotein. (F) The graph represents the level of low-density lipoprotein. (G) The graph represents the ratio of low-density lipoprotein to high-density lipoprotein. (H) The graph represents the level of fasting blood sugar. Bottom and top lines of graph show minimum and maximum score, respectively. The bottom, middle, and top lines of the box represent the 25th, 50th, and 75th percentiles, respectively. The length of the box indicates the interquartile range. Whisker represent distance of minimum and maximum score from the bottom and top lines of the box, this distance is less than 1.5 times the length of the box (or < 1.5 × interquartile range). *: P < 0.05; **: P < 0.01; ***: P < 0.001 vs. baseline; #: P < 0.05; ##: P < 0.01; ###: P < 0.001 vs. first month.
The pairwise comparison of ALT levels at different times in the 2 groups was significant (P < 0.01). No significant difference was detected in the pairwise comparison of the TG, cholesterol, and LDL values at different times in the control group (P > 0.05). However, in the intervention group, all times had significant differences (P < 0.001). According to the post-hoc results, no significant difference was detected in the HDL levels in the control group (P > 0.05). The intervention group showed no significant difference in the HDL levels at baseline and in the first month (P = 0.35), whereas a significant difference was detected in baseline and second-month comparison and the first- and second-month comparison (P < 0.05). The pairwise comparison of the changes in the LDL/HDL parameter in the control group did not have a significant difference at any time (P > 0.05), while this comparison was significant within the intervention group at all times (P < 0.001). The difference in the amount of FBS in the control group was not significant in the post-hoc test (P > 0.05). In the intervention group, the difference in the FBS level was not significant at baseline compared to the first (P = 0.51) and second month (P = 0.07), but it was significant in the first and second month (P = 0.02; Figure 2).
5. Discussion
We assessed the GSE effects in NAFLD patients. The major findings of this study indicated that GSE administration for 2 months could improve liver aminotransferase enzymes (AST and ALT) and lipid profiles (LDL, cholesterol, HDL, TG, and LDL/HDL) and FBS in fatty liver patients.
Obesity is considered the main risk factor in NAFLD; thus, a low-carbohydrate and low-calorie diet is suggested in the initial stage of the disease (13). The data showed that 200-mg GSE twice a day for 2 months did not affect weight and BMI in patients. The pathogenesis of NAFLD as a metabolic disorder results from a complex interaction between hormonal, nutritional, and genetic factors. Some factors, such as obesity, dyslipidemia, and insulin resistance, play a role in the pathogenesis of NAFLD (13-15).
NAFLD is an asymptomatic increase in liver function tests. The liver enzymes exhibit any abnormality pattern, but ALT or AST levels are elevated in more than 70% of cases (16). In this clinical trial, the serum ALT and AST levels decreased significantly in the GSE group compared to the placebo group over time. The serum levels of ALT and AST at various times (baseline, first month, and second month) were significant in the GSE group.
GSEs are rich in flavonoids (especially in Pas) and have strong antioxidant effects. Orally administered GSE lowered reactive oxygen species (ROS) formation and protected plasma protein carbonyl groups, whereas it increased the endogenous antioxidant system activity (17-20). The antioxidant effect of GSE have been confirmed in clinical trials (17). Scientists believe that phytochemicals can protect cells from the effect of unstable oxygen leading to the prevention against the occurrence of disease (21). Grape seeds have can protect against oxidative damage to DNA (18, 22). Grape seed PAs possess antioxidant effects 20 times more compared to vitamin C and 50 times more compared to vitamin E (23).
Ali et al. indicated the hepatoprotective effect of seed and skin extract of grape against Ehrlich solid tumor- related oxidative stress in mice; they showed that the antioxidant effect of them protected hepatocytes against tumor and decreased serum liver aminotransferase enzymes in mice (24). Hemmati et al. compared the anti-fibrogenic effect of GSE to vitamin E as a usual antioxidant; they showed the anti-fibrogenic effect of GSE via alternation in the malondialdehyde (MDA) level (25).
Therefore, the ability of GSE to decrease liver enzymes can be attributed to its antioxidant properties. However, more investigations must be performed to verify the involved mechanism. Previous studies have demonstrated some clinical effects of GSE due to their antioxidant properties (18-20), including anticancer (26, 27), antimicrobial (28), anti-inflammatory properties (29, 30), as well as the activation of the apoptosis signal (28). Our findings confirm those of many experimental studies, approving the beneficial GSE effects as an antioxidant substance.
Dyslipidemia can also cause NAFLD. The NAFLD prevalence in dyslipidemia patients is as high as 50% (31). Therefore, correction of dyslipidemia, especially hypertriglyceridemia has been considered for the management of NAFLD/NASH based on the guidelines (32). Here, GSE could improve lipid profiles and significantly decreased serum TG and cholesterol concentrations in patients. The LDL/HDL ratio was reduced as the best single predictor of cardiovascular disease (33). Thus, the GSE supplementation impact on LDL/HDL cholesterol in cases with NAFLD can be useful, particularly in cardiovascular patients. The amount of HDL significantly increased after 2 months compared to baseline and first month in patients who received GSE. On the other hand, based on these results, using of GSE 200 mg, twice a day for 2 months can be effective in restoring lipid profiles in fatty liver patients. Natural compounds that have great polyphenols, like phenolic acids (chlorogenic and neochlorogenic acids) and flavonoids (PAs, anthocyanins, flavonols and flavanols), exhibited beneficial effects, like strong antioxidant effect and potential therapeutic and medicinal advantageous (hepatoprotective, gastroprotective, antiproliferative, or anti-inflammatory activities) (34, 35). They can also be beneficial in preventing chronic diseases, such as diabetes, metabolic disorders, and cardiovascular diseases due to their supportive effects on lipid profiles, FBS, and blood pressure concentrations. Also, some mechanisms may be associated with lowering lipid profiles of these compounds with a high amount of cyanide and proanthocyanidins, including action influence on peroxidation, lipid metabolism, inflammation process, oxidation and coagulation (36, 37). Charradi et al. indicated that GSE protected the liver against fat-related lipotoxicity and protects the liver function against high-fat diet-induced liver steatosis; they showed that GSE decreased all hepatic lipid contents (38).
Insulin resistance can be an underlying mechanism associating NAFLD with type two diabetes, metabolic syndrome and obesity. Hence, increasing glycemic control and insulin sensitivity is useful for the control of NAFLD, NASH, and other associated comorbidities (14). A significant reduction was observed in FBS levels after receiving of GSE after 2 months. Herbals with rich sources of anthocyanins (such as black chokeberry [Aronia melanocarpa]) may prevent obesity, which is linked to a reduction in lipids and sugars absorption in the digestive system (34). It seems that bioactive compounds such as anthocyanins and proanthocyanidine could decrease FBS in GSE.
According the results of this study and previous studies, natural compounds with proantocyanidine could be beneficial potential role in treatment of some disease that related to stress oxidative process. However, their efficacy and toxicity must be evaluated in the future studies.
This study, like many others, had some limitations. First, it was impossible to conduct this trial on the severe fatty liver patients, so efficacy of GSE on the severe NAFLD is unclear. Second, because of unknown effects on GSE on other organs, it is suggested that probable effects of GSE, investigated. We also suggest measuring the level of superoxide dismutase (SOD) and MDA to evaluate the exact antioxidant mechanism of GSE. To get sustainable results, such supplement needs to be used for a longer period of time. In such a situation, we may get a better profile of the factors involved in NAFLD.
In conclusion, the findings of these studies clarified that GSE with antioxidant and anti-inflammatory properties had enough potential to be considered as a supplement for the management and treatment of NAFLD.