1. Background
Hepatitis B Virus (HBV) infection, as a common disease, can be effectively treated by blocking viral replication. Interferons (IFNs) and nucleotide drugs have been applied to treat HBV (1). Among them, IFNs can block viral replication by exerting direct antiviral activity and augmenting immunity simultaneously, with obvious therapeutic effects (2). However, the clinical use of IFNs is limited due to many contraindications, side effects, and the need for intramuscular injection. Represented by tenofovir and entecavir (ETV), nucleoside drugs that can be taken orally have been widely used to treat HBV. ETV blocks the replication of HBV in vivo mainly by inhibiting HBV polymerase and relieves the damage of immune response to hepatocytes (3). Nevertheless, whether ETV is helpful in the recovery of T cell immune function remains unclear. Patients are less resistant to ETV than to other anti-HBV drugs, and ETV is also suitable for those who have no response to tenofovir treatment.
HBV pregenomic RNA (pgRNA) is the translation template of HBV core antigen and polymerase protein and the reverse transcription template of HBV, so it plays an essential role in the life cycle of HBV (4). The serum level of pgRNA can reflect the effect of antiviral therapy on HBV (5). Besides, this level has been extensively utilized to guide rational drug use (6, 7). Until now, the influence of ETV on patients with HBV infection has never been evaluated in terms of HBV-DNA, IFN-γ, or pgRNA.
2. Objectives
This study aimed to assess the effects of ETV on serum HBV-DNA, IFN-γ, and pgRNA in patients with infection and provide valuable evidence for effective HBV treatment.
3. Methods
3.1. General Data
The clinical data of 300 HBV patients admitted from January 2017 to January 2019 were retrospectively analyzed, of whom 193 cases administered with ETV were assigned to an observation group, and the remaining 107 untreated cases (who refused treatment) were assigned to a blank control group. In the observation group, there were 104 males and 89 females aged 19 - 49 years, with an average of 34.58 ± 6.41 years, and the mean duration of disease was 3.12 ± 1.24 years. In the blank control group, there were 59 males and 48 females aged 20 - 49 years, with an average of 34.83 ± 6.82 years, and the mean disease duration was 2.98 ± 1.33 years. The general data of the two groups were comparable (P > 0.05). The hospital ethics committee approved this study.
3.2. Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) patients meeting the diagnostic criteria for hepatitis B e antigen (HBeAg) (+) hepatitis B (8), (2) those without liver cirrhosis, liver cancer, drug-induced liver injury or alcoholic liver disease, (3) those with good compliance and ability to take medication and visit regularly, and (4) those who signed the informed consent form. The exclusion criteria were as follows: (1) patients complicated with hepatitis C or hepatitis D, (2) pregnant or lactating women, (3) those with dysfunction of heart, brain, kidney, or other vital organs, or (4) those with HIV infection.
3.3. Treatment Methods
The observation group was given ETV Tablets (NMPN H20080798, Sino-American Shanghai Squibb Pharmaceuticals Ltd., 1.0 mg) at a dose of 0.5 mg/d. The blank control group received no drug administration. The treatment lasted for one year.
3.4. Serum Test
Before treatment and 12, 24, and 48 weeks after treatment, 3 - 4 mL of peripheral venous blood was collected from each patient in our hospital, left still at room temperature for 20 min, and centrifuged at 3000 r/min for 15 min. The supernatant was added into 1.5 mL EP tubes and stored at -80°C. Then the liver function [aspartate aminotransferase (AST), and alanine aminotransferase (ALT)], HBV serum markers [hepatitis B surface antigen (HBsAg), and HBeAg], and T helper 1 (Th1) cytokine (IFN-γ) were detected by Enzyme-linked Immunosorbent Assay (ELISA) using an AU5400 biochemical analyzer (Olympus, Japan) and ELISA kits (Wuhan USCN Sciences Co., Ltd., China).
3.5. Detection of HBV-DNA Content
The HBV-DNA content was detected by quantitative PCR using COBAS AmpliPrep/COBAS TaqMan automatic virus quantification system (Roche, Switzerland; detection range for HBV-DNA: 2.293×103 - 7.566×109 copies/mL), ABI real-time quantitative PCR system (Bio-Rad, USA), and HBV cDNA synthesis kit (Bio-Rad, USA). HBV-DNA upstream primer was 5'-ATACTGCGGAACTCCTACG-3,' and the downstream primer was 5'-CCGCGTAAAGAGAGGTGGC-3'. PCR mixture contained 37.6 µL of HBV-PCR solution, 0.4 µL of Taq polymerase, and 0.06 µL of the specimen or positive template. PCR conditions were as follows: pre-denaturation at 37°C for 5 min, denaturation at 94°C for 60 s, annealing at 95°C for 5 s, and extension at 60°C 30 s, 40 cycles in total.
3.6. Detection of HBV pgRNA Content
The HBV pgRNA content was detected by a nucleic acid kit developed by the School of Basic Medicine, Peking University (China), and the same apparatus as that for HBV-DNA measurement, with HBV pgRNA upstream primer: 5'-TTAAC-GACCAGGATAGTCTGAC-3' and a downstream primer: 5'-CT-TAGCCTAGGCTTTGACATGA-3'. PCR mixture contained 38 µL of the reaction solution, 0.2 µL of internal standard, and 2 µL of an enzyme mixture. PCR conditions were as follows: denaturation at 50°C for 2 min, at 94°C for 5 min, and at 94°C for 15 s, annealing at 57°C for 30 s, and extension at 57°C for 30 s, 45 cycles in total. The primers were purchased from Wuhan Boster Biological Technology Co., Ltd. (China).
3.7. Observation Indicators
The two groups' liver function, HBV serum markers, IFN-γ, HBV-DNA, and HBV pgRNA were compared before treatment and 12, 24, and 48 weeks after treatment. The negative conversion rates of HBeAg and HBV-DNA were compared 12, 24, and 48 weeks after treatment. The adverse drug reactions during treatment were recorded, including headache, fatigue, dizziness, and nausea caused by ETV.
3.8. Statistical Analysis
SPSS 23.0 software (IBM Inc., USA) was used for statistical analysis. The measurement data were described as mean ± standard deviation (
4. Results
4.1. Liver Function and HBV Serum Markers
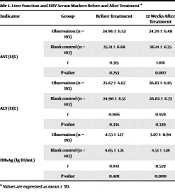
There were no significant differences in the levels of AST and ALT between the observation and blank control groups before and after treatment (P > 0.05). The level of HBsAg did not differ significantly between the observation and blank control groups before treatment (P > 0.05), while it was lower in the observation group than in the blank control group 12, 24, and 48 weeks after treatment (P < 0.05) (Table 1).
| Indicator | Group | Before Treatment | 12 Weeks After Treatment | 24 Weeks After Treatment | 48 Weeks After Treatment | F | P-Value |
|---|---|---|---|---|---|---|---|
| AST (U/L) | Observation (n = 193) | 24.96 ± 6.52 | 24.70 ± 6.48 | 26.11 ± 6.92 | 25.86 ± 6.71 | 2.029 | 0.108 |
| Blank control (n = 107) | 25.21 ± 6.68 | 26.14 ± 6.75 | 24.93 ± 6.50 | 26.59 ± 6.86 | 1.445 | 0.229 | |
| t | 0.315 | 1.816 | 1445 | 0.895 | |||
| P-value | 0.753 | 0.007 | 0.150 | 0.372 | |||
| ALT (U/L) | Observation (n = 193) | 25.67 ± 6.67 | 26.83 ± 6.95 | 27.21 ± 7.02 | 26.52 ± 6.83 | 1.758 | 0.154 |
| Blank control (n = 107) | 24.90 ± 6.51 | 26.02 ± 6.73 | 26.45 ± 6.81 | 24.99 ± 6.71 | 1.403 | 0.241 | |
| t | 0.966 | 0.978 | 0.908 | 1.870 | |||
| P-value | 0.335 | 0.329 | 0.365 | 0.062 | |||
| HBsAg (lg IU/mL) | Observation (n = 193) | 4.53 ± 1.17 | 3.07 ± 0.94 | 2.57 ± 0.76 | 1.82 ± 0.52 | 325.605 | 0.000 |
| Blank control (n = 107) | 4.65 ± 1.21 | 4.51 ± 1.18 | 4.84 ± 1.30 | 4.60 ± 1.20 | 1.387 | 0.246 | |
| t | 0.841 | 11.579 | 19.090 | 27.839 | |||
| P-value | 0.401 | 0.000 | 0.000 | 0.000 |
a Values are expressed as mean ± SD.
4.2. Serum IFN-γ, HBV-DNA and HBV pgRNA
There were no significant differences in the levels of IFN-γ, HBV-DNA, and HBV pgRNA between the observation and blank control groups before treatment (P > 0.05). The levels of IFN-γ, HBV-DNA, and HBV pgRNA in the observation group were lower than those in the blank control group 12, 24, and 48 weeks after treatment (P < 0.05) (Table 2).
| Indicator | Group | Before Treatment | 12 Weeks After Treatment | 24 Weeks After Treatment | 48 Weeks After Treatment | F | P-Value |
|---|---|---|---|---|---|---|---|
| IFN-γ (pg/mL) | Observation (n = 193) | 38.62 ± 10.27 | 18.07 ± 4.58 | 10.52 ± 2.46 | 10.16 ± 2.14 | 1005.068 | 0.000 |
| Blank control (n = 107) | 39.20 ± 10.64 | 39.51 ± 10.15 | 40.26 ± 10.91 | 39.93 ± 10.51 | 0.208 | 0.891 | |
| t | 0.463 | 25.116 | 36.287 | 38.003 | |||
| P-value | 0.644 | 0.000 | 0.000 | 0.000 | |||
| HBV-DNA (lg IU/mL) | Observation (n = 193) | 7.30 ± 2.43 | 3.20 ± 1.21 | 2.64 ± 1.03 | 2.24 ± 0.81 | 463.945 | 0.000 |
| Blank control (n = 107) | 7.18 ± 2.28 | 7.49 ± 2.43 | 7.61 ± 2.55 | 7.10 ± 2.26 | 1.121 | 0.340 | |
| t | 0.419 | 20.402 | 23.821 | 26.944 | |||
| P-value | 0.676 | 0.000 | 0.000 | 0.000 | |||
| HBV pgRNA (lg copy/mL) | Observation (n = 193) | 6.50 ± 2.26 | 3.35 ± 1.06 | 2.49 ± 0.84 | 2.30 ± 0.71 | 393.569 | 0.000 |
| Blank control (n = 107) | 6.64 ± 2.29 | 6.97 ± 2.45 | 6.21 ± 2.08 | 6.87 ± 2.32 | 2.332 | 0.074 | |
| t | 0.512 | 17.763 | 21.860 | 25.338 | |||
| P-value | 0.609 | 0.000 | 0.000 | 0.000 |
a Values are expressed as mean ± SD.
4.3. Negative Conversion Rates of HBeAg and HBV-DNA
The HBeAg and HBV-DNA negative conversion rates of the observation group were higher than those of the blank control group 12, 24, and 48 weeks after treatment (P < 0.05) (Table 3).
| Group | HBeAg | HBV-DNA | ||||
|---|---|---|---|---|---|---|
| 12 Weeks After Treatment | 24 Weeks After Treatment | 48 Weeks After Treatment | 12 Weeks After Treatment | 24 Weeks After Treatment | 48 Weeks After Treatment | |
| Observation (n = 193) | 7 (3.63) | 11 (5.70) | 16 (8.29) | 18 (9.33) | 26 (13.47) | 31 (16.06) |
| Blank control (n = 107) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| χ2 | 0.974 | 6.331 | 9.370 | 10.616 | 15.782 | 19.167 |
| P-value | 0.046 | 0.012 | 0.002 | 0.001 | 0.010 | 0.000 |
a Values are expressed as No. (%).
4.4. Adverse Reactions
During treatment, the observation group's incidence rate of adverse events was 2.07% (4/193), including two cases of headache, one case of dizziness, and one case of nausea. The symptoms were remitted without intervention one day later.
5. Discussion
The life cycle of HBV consists of the following steps. HBV binds the sodium taurocholate cotransporting polypeptide receptor and other receptors. Relaxed circular DNA (rcDNA) enwrapped by nucleocapsid enters hepatocytes and is delivered to the nucleus following nucleocapsid uncoating and nuclear translocation. Then, rcDNA is converted into HBV covalently closed circular DNA (cccDNA) as the transcription template of HBV RNA and the translation template of HBV core antigen and polymerase protein. PgRNA (3.5 kb) binds P protein with reverse transcription activity into a complex that then enters the viral capsid. rcDNA is generated by the reverse transcription of pgRNA. Newly generated viral particles are secreted through the endoplasmic reticulum to produce a complete progeny virus (9, 10). Thus, cccDNA is the "source" of HBV replication, and the transcription of pgRNA is only derived from cccDNA, suggesting that pgRNA can reflect the activity of cccDNA as a biomarker of HBV (11). Xiang et al. reported that the serum pgRNA level of HBV patients was correlated with the levels of HBV-DNA, HBsAg, ALT, and AST (12). In addition, Luo et al. found that serum pgRNA was an independent marker for predicting the HBeAg seroconversion and virological response in HBV patients (13).
The currently available nucleotide analogs can prevent HBV from entering cells for continuous replication and release by inhibiting the reverse transcription of pgRNA, thereby keeping the stability of the cccDNA library (14). ETV is a nucleoside analog that suppresses HBV-DNA polymerase activity and prevents viral replication by competing for deoxyadenosine diphosphate substrate and terminating viral DNA strand synthesis (15). As an epoxy hydroxy carbon deoxyguanosine analog, ETV can inhibit the initiation activity of HBV polymerase, pgRNA negative-strand synthesis, and HBV-DNA positive-strand by competing for deoxyguanosine triphosphate, the natural substrate of HBV polymerase (16). In this study, the levels of HBsAg, HBV-DNA, and HBV pgRNA in the observation group were lower than those in the blank control group 12, 24, and 48 weeks after treatment, indicating that ETV had a noticeable anti-HBV effect. Possibly, ETV can be phosphorylated more effectively than other nucleoside analogs, so it has a higher anti-HBV activity (17). For example, ETV can be absorbed by hepatocytes and phosphorylated into mono-, di- and triphosphates, inhibiting the actions of viral reverse transcriptase and exerting a more substantial anti-HBV effect (18).
However, the HBeAg and HBV-DNA negative conversion rates currently remain unsatisfactory in clinical practice. In this study, the HBeAg and HBV-DNA negative conversion rates of the observation group were higher than those of the blank control group 12, 24, and 48 weeks after treatment. Nevertheless, ETV may sometimes not work well due to the ALT normalization and reduced viral replication, tissue damage, and necroinflammation during antiviral therapy (19). Persistent elevation of ALT level is considered a risk factor for concomitant diseases such as steatosis and cardiovascular disease and is associated with mild regression of HBV-related cirrhosis (20). Geng et al. found that ETV was the most effective nucleotide analog for ALT normalization during HBV treatment (21). In this study, AST and ALT levels had no significant changes in the observation and blank control groups before and after treatment, indicating that ETV helped maintain the stability of liver function.
The progression and outcome of HBV infection are closely related to the intensity of the antiviral immune response. Gu et al. confirmed a relationship between the serum HBV pgRNA and the host immunity of HBV patients (22). HBV clearance primarily depends on cellular immunity involving T lymphocytes, NK cells, and macrophages and their activated non-specific inflammatory factors (23). Th1 cytokines play an adjuvant role in cytotoxic T cells and benefit viral clearance in HBV infection. As the most representative Th1 cytokine, IFN-γ can regulate immunity by activating and enhancing the activity of T cells, NK cells, and macrophages (24). Huang et al. reported that IFN-γ was a potential biological indicator for the early prediction of HBV reactivation (25). However, IFN-γ not only promotes viral clearance but may also cause an excessive immune response and liver damage, so an abnormally elevated IFN-γ level does not necessarily mean liver inflammation and necrosis can be avoided. In this study, the level of IFN-γ in the observation group was lower than that in the blank control group 12, 24, and 48 weeks after treatment, suggesting that ETV may be implicated in the anti-HBV immune response. After ETV treatment, viruses were possibly suppressed, and cellular immune response was enhanced simultaneously. With the decrease of HBeAg and HBV-DNA load, the Th1 cytokine response was up-regulated, and the T cell viability was restored by overcoming the low response of cytotoxic T cells upon HBV, thereby enhancing the anti-HBV immunity and providing favorable conditions for viral clearance (7).
During treatment, HBV-DNA replication was continuously inhibited, and immune response was regulated accordingly, reducing the IFN-γ level and thus controlling the onset and progression of liver inflammation and fibrosis. Furthermore, we herein found that the adverse reactions were mild in the observation group during treatment, suggesting the high safety of ETV. However, this study has limitations. This is a single-center study with a small sample size. Additionally, this is a retrospective study. Hence, multicenter prospective studies with larger sample sizes must validate our findings.
In conclusion, antiviral therapy with ETV can inhibit the replication of HBV-DNA, increase the HBV-DNA negative conversion rate, enhance immune function, and reduce the expression of HBV pgRNA in HBV patients.