1. Background
With the improvement in living standards and the change in eating habits, the prevalence of non-alcoholic fatty liver disease (NAFLD) has gradually increased. It has become one of the most common chronic liver diseases worldwide. The prevalence rate in Asian countries is about 25% (1), while in Chinese adults, the NAFLD prevalence rate is 29.2% (2). NAFLD is a metabolic liver injury closely related to insulin dysfunction and genetics. It is a liver disease caused by excessive lipid deposition in liver cells caused by other known liver injury factors, excluding alcohol (3). NAFLD causes liver damage and impacts cardiovascular, cerebrovascular, endocrine, and urinary systems (4), leading to a significantly increased risk of cardiovascular and cerebrovascular diseases, type 2 diabetes, and chronic kidney disease.
Based on the severity, NAFLD can be divided into nonalcoholic simple fatty liver, nonalcoholic steatohepatitis (NASH), liver cirrhosis, and hepatocellular carcinoma (5). Non-alcoholic simple fatty liver is usually regarded as a benign nonprogressive disease. NASH can gradually develop into liver fibrosis, cirrhosis, and even hepatocellular carcinoma, so NASH has gradually become a hot and difficult research topic in recent years.
Epidemiological data support the association between vitamin D deficiency and NAFLD. Vitamin D is an essential fat-soluble vitamin for the human body and plays a biological role by acting on the vitamin D receptor (VDR) in cells (6). Population studies have found that vitamin D deficiency is a risk factor for NAFLD (7). Experimental studies suggest that the vitamin D/VDR axis may be involved in the pathogenesis and progression of NAFLD, linking vitamin D-mediated metabolic pathways to key processes leading to hepatic steatosis, inflammation, and fibrosis (8). Indeed, vitamin D may influence the development of NAFLD in direct and indirect ways (9). In a rat model of NAFLD, active vitamin D treatment reduces hepatic inflammation and oxidative stress by inhibiting cellular senescence (10). The documented associations between vitamin D deficiency and NAFLD suggest that vitamin D supplementation may be a potential therapeutic option for NAFLD in children (11) and adults (12). Therefore, investigating the incidence of NAFLD and the mechanism by which vitamin D ameliorates NAFLD can illuminate the NAFLD pathogenesis pathways and provide a theoretical basis for the pathway by which vitamin D ameliorates NAFLD.
2. Objectives
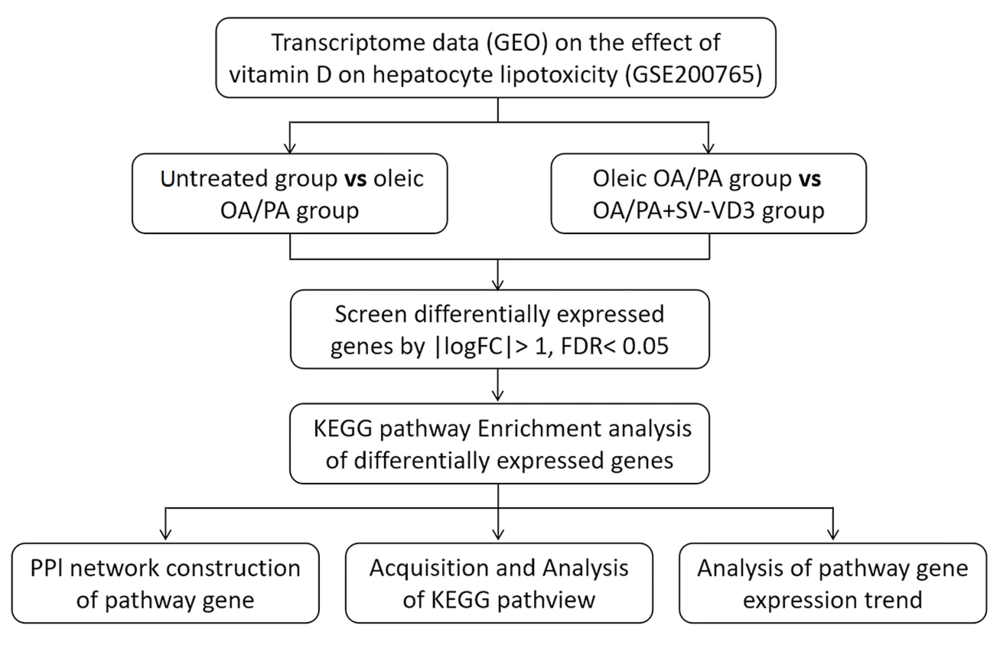
To gain insight into the mechanisms of vitamin D regulation of hepatic steatotoxicity in NAFLD patients, this study analyzed the GEO database of vitamin D intervention in hyperlipidemia-induced NAFLD cell models. By analyzing differentially expressed genes between the control group, NAFLD cell model, and vitamin D3 intervention group, it was possible to find out how vitamin D regulates the epigenetic properties of genes in hepatocytes and thus ameliorates steatotoxicity. The results of this study may have an important role in revealing the role of vitamin D in regulating liver function and preventing or treating NAFLD. At the same time, this study provides a new perspective on the epigenetic regulation mechanisms of genes, which provides important clues for an in-depth investigation of the pathogenesis of NAFLD.
3. Methods
3.1. Public Data Collection
The National Biotechnology Information Center (NCBI) gene expression comprehensive database GEO is a functional genomics database for high-throughput gene expression chip and micro-array data (13). The expression analysis data chip (GSE200765) of vitamin D's cytoprotective effect on human hepatocyte lipotoxicity was screened from the database. Three HepaRG cells untreated, three HepaRG cells pre-treated only with a mix of oleic OA/PA, and three HepaRG cells treated with SV-VD3 and OA/PA were utilized as the research objects.
3.2. Data Screening and Processing
GEO2R, an online analysis tool included in the GEO database, is an interactive webpage tool (14). It can compare two or more data sets in the GEO series to determine differentially expressed genes under experimental conditions (15). In the GSE200765 dataset, untreated HepaRG cells were named as an untreated group, HepaRG cells pretreated with oleic acid OA/PA mixture were named as oleic OA/PA group, and Hep ARG cells treated with SV-VD3 and OA/PA were named as OA/PA+SV-VD3 group. GEO2R was used to screen differentially expressed genes in (untreated) vs. (oleic OA/PA) and (oleic OA/PA) vs (OA/PA + SV-VD3). The screening criteria for differentially expressed genes were P < 0.05 and |logFC|>1. The genes with log FC > 1 were up-regulated differentially expressed, and those with log FC<-1 were down-regulated differentially expressed. Volcano maps were used to visualize the differential distribution of genes and screen for differentially expressed genes.
3.3. Enrichment Analysis of Differential Gene Pathway
The Kyoto Encyclopedia of Genes and Genes (KEGG) pathway enriched for differentially expressed genes was analyzed using the DAVID database. The differentially expressed genes were input into the gene list, with the identifier selected as the official gene symbol and the species selected as human, and then uploaded. After obtaining the KEGG pathway enriched by differentially expressed genes, screening was performed using FDR<0.05 as the standard.
3.4. Analysis of Pathway Gene Interaction
The screened pathway genes were imported into the STRING database, and the organism selected was Homo sapiens. After selecting a minimum interaction score of 0.400 and removing unconnected targets, the protein-protein interaction (PPl) network was predicted and constructed.
3.5. Analysis of Expression Trend of Pathway Genes
After obtaining the key pathway view from the KEGG database, the upregulation or downregulation expression trends of differentially expressed genes were marked in the graph. Then, a t-test or unpaired t-test with Welch's correction was used to analyze the expression trend of the pathway genes. We have depicted and described the research process in Figure 1.
4. Results
4.1. Differential Expression Gene Analysis
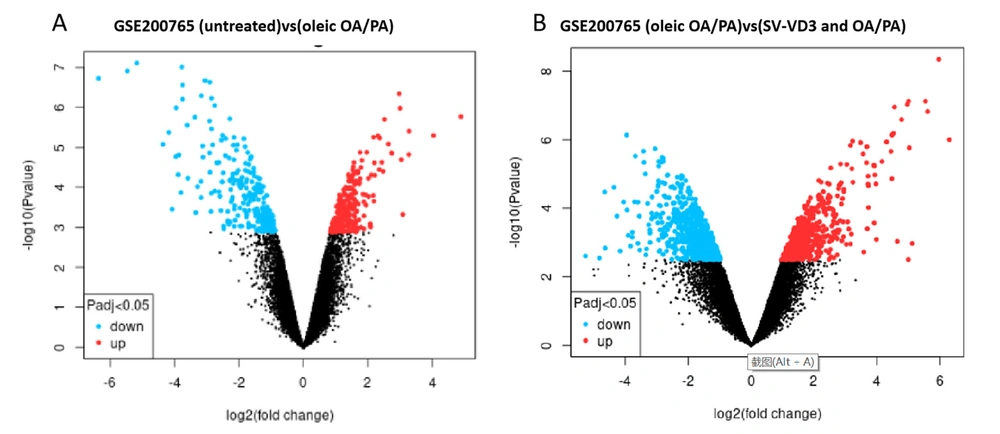
Differentially expressed genes between HepaRG cells untreated and HepaRG cells pre-treated with a mix of oleic OA/PA were screened by the GEO2R tool (Figure 2A). At the same time, we screened the differentially expressed genes between HepaRG cells pre-treated with a mixture of oleic OA/PA and HepaRG cells treated with OA/PA and SV-VD3 (Figure 2B). After the oleic OA/PA was added to HepaRG cells, 218 genes were up-regulated, and 255 genes were down-regulated. After SV-VD3 was added to HepaRG cells treated with OA/PA, 554 genes were up-regulated, and 704 were down-regulated.
Transcriptome differences of vitamin D in lipotoxicity of human hepatocytes in GSE200765 sequencing chip. Red dots represent differentially expressed up-regulated genes, blue dots represent expressed down-regulated genes, and black dots represent no significant difference in gene expression. (A) Two hundred eighteen upregulated genes and 255 downregulated genes were screened between the untreated HepaRG and the HepaRG cell group treated with only oleic acid OA/PA mixture. (B) 554 upregulated genes and 704 downregulated genes were screened and compared between the HepaRG cell group treated with OA/PA and the HepaRG cell group treated with SV-VD3 and OA/PA. OA/PA: oleic acid/permitic acid. SV-VD3: synthetic vitamin-vitamin D3. The screening criteria for differentially expressed genes were P < 0.05 and |logFC|>1.
4.2. KEGG Pathway Analysis
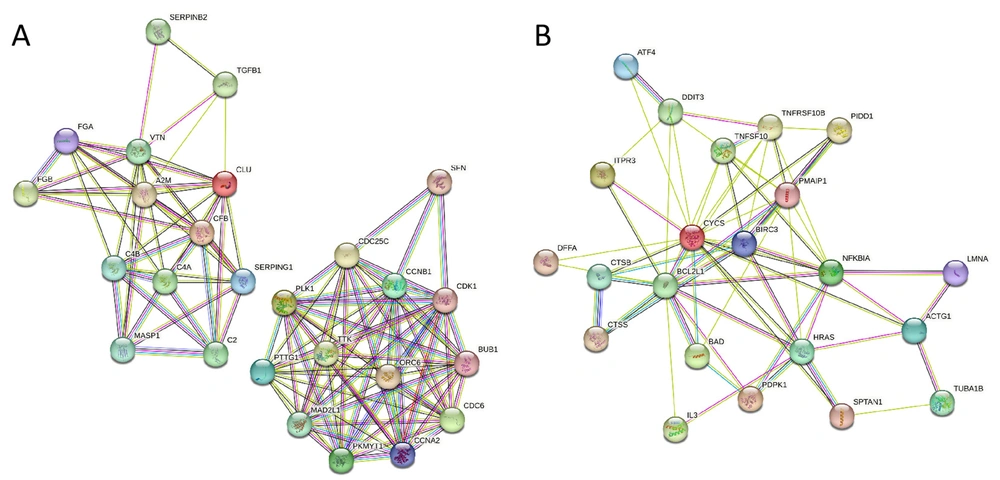
The DAVID database was used to enrich the KEGG pathway for differentially expressed genes (Table 1). After oleic OA/PA was added to HepaRG cells, the differential expressed genes were enriched in the complex coagulation cascades and cell cycle pathways. After SV-VD3 was added to the OA/PA pre-treated HepaRG cells, the differential expressed genes were enriched in the apoptosis pathway. Two closely interacting protein networks were formed between the differentially expressed pathway genes of the (untreated) group and (OA/PA) group (Figure 3A). Differentially expressed pathway genes of (the OA/PA) group, and (SV-VD3 and OA/PA) group formed a closely interacting protein network (Figure 3B).
| Category | Groups and Terms | Description | Count | FDR |
|---|---|---|---|---|
| KEGG Pathway | (untreated) vs. (OA/PA) | |||
| hsa04610 | Complement and coagulation cascades | 12 | 0.002279391 | |
| hsa04110 | Cell cycle | 14 | 0.002279391 | |
| (OA/PA) vs (SV-VD3 and OA/PA) | ||||
| hsa04210 | Apoptosis | 23 | 0.025568275 |
The interaction network of different groups of KEGG pathway genes. (A) The protein interaction network between 26 pathway genes screened in the (untreated) vs. (OA/PA) group. (B) The protein interaction network between 23 pathway genes screened in the (OA/PA) vs. (SV-VD3 and OA/PA) group. The images were analyzed and obtained from the STRING database.
4.3. Expression Trend of Pathway Genes Supplemented with SV-VD3
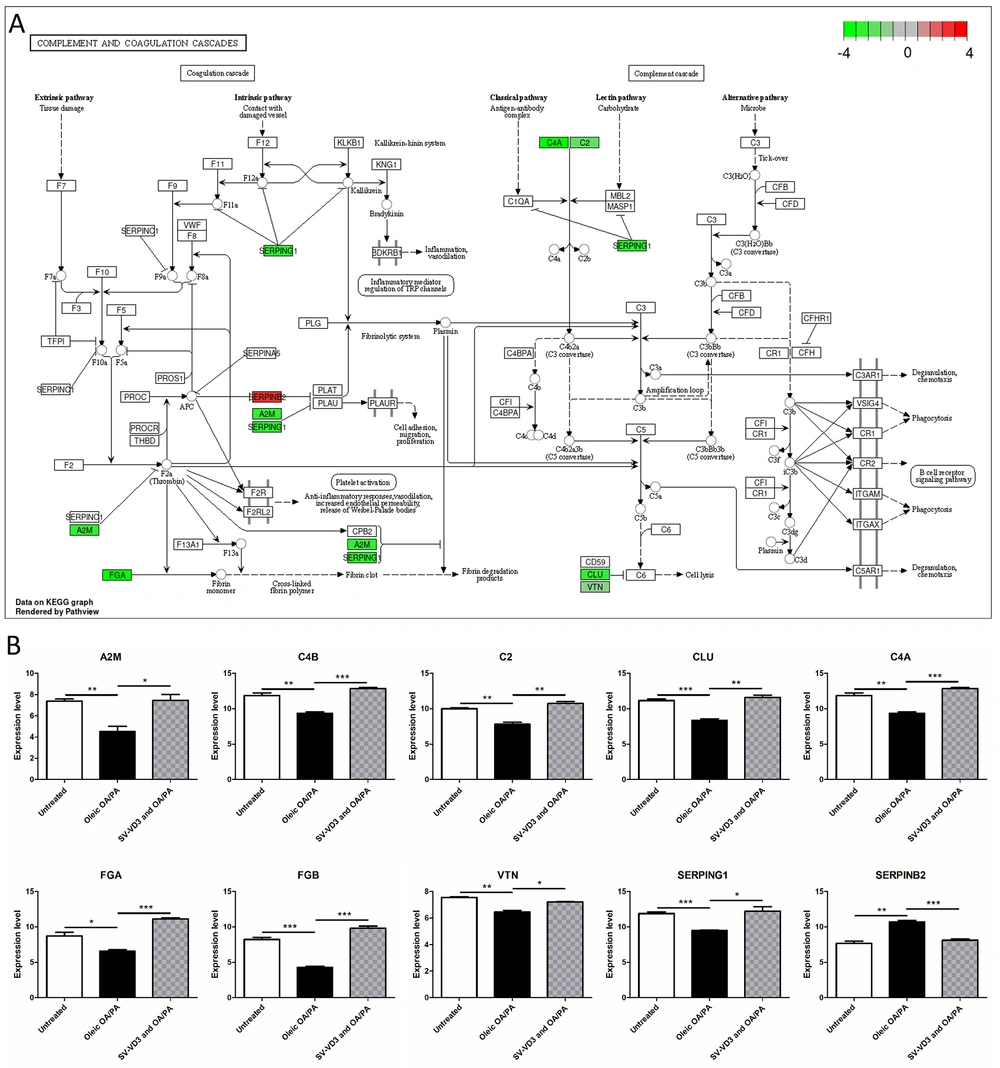
After the HepaRG cells were pre-treated with oleic acid OA/PA mixture, the expression of C4B (P = 0.0034), FGB (P = 0.0003), C4A (P = 0.0034), FGA (P = 0.0160), VTN (P = 0.0015), SERPING1 (P = 0.0004), A2M (P = 0.0058), CLU (P = 0.0008) and C2 (P = 0.0023) in the complement and coagulation cascades pathway was down-regulated, and the expression of SERPINB2 (P = 0.0017) was up-regulated (Figure 4A and B). After SV-VD3 was added on this basis, the expression of the above genes was reversed (Figure 4B).
Vitamin D improves liver cell lipid toxicity by regulating the complement and coagulation cascade pathways. (A) HepaRG cells pre-treated with a mix of oleic OA/PA lead to abnormal gene expression of complement and coagulation cascades pathways. The image reference source is the KEGG pathway database (16). The red gene represents upregulation, while the green gene represents downregulation. (B) The changing trend of complement and coagulation cascades pathway genes under three treatments. *P < 0.05,**P < 0.01,***P < 0.001.
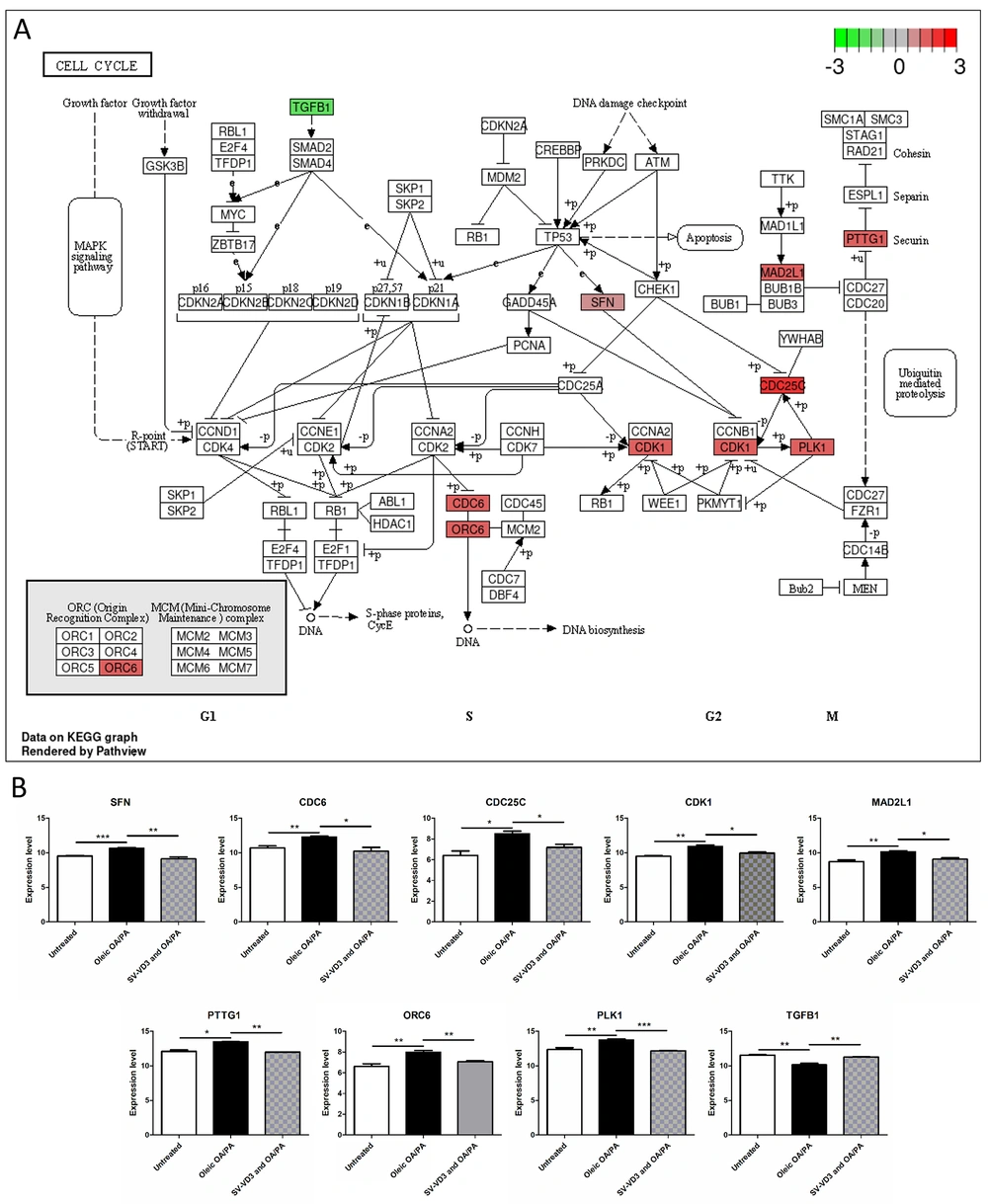
After the HepaRG cells were pre-treated with oleic acid OA/PA mixture, the expression of PLK1 (P = 0.0099), CDC6 (P = 0.0078), CDC25C (P = 0.0142), ORC6 (P = 0.0089), PTTG1 (P = 0.0270), CDK1 (P = 0.0048), SFN (P = 0.0009) and MAD2L1 (P = 0.0070) in the cell cycle pathway was up-regulated, and the expression of TGFB1 (P = 0.0051) was down-regulated (Figure 5A and B). After SV-VD3 was added on this basis, the expression of the above genes was reversed (Figure 5B).
Vitamin D improves liver cell lipid toxicity by regulating the cell cycle pathway. (A) HepaRG cells pre-created with a mix of oleic OA/PA led to abnormal gene expression of the cell cycle pathway. The image reference source is the KEGG pathway database (16). The red gene represents up-regulation, while the green gene represents down-regulation. (B) The changing trend of cell cycle pathway genes under three treatments. *P < 0.05,**P < 0.01,***P < 0.001.
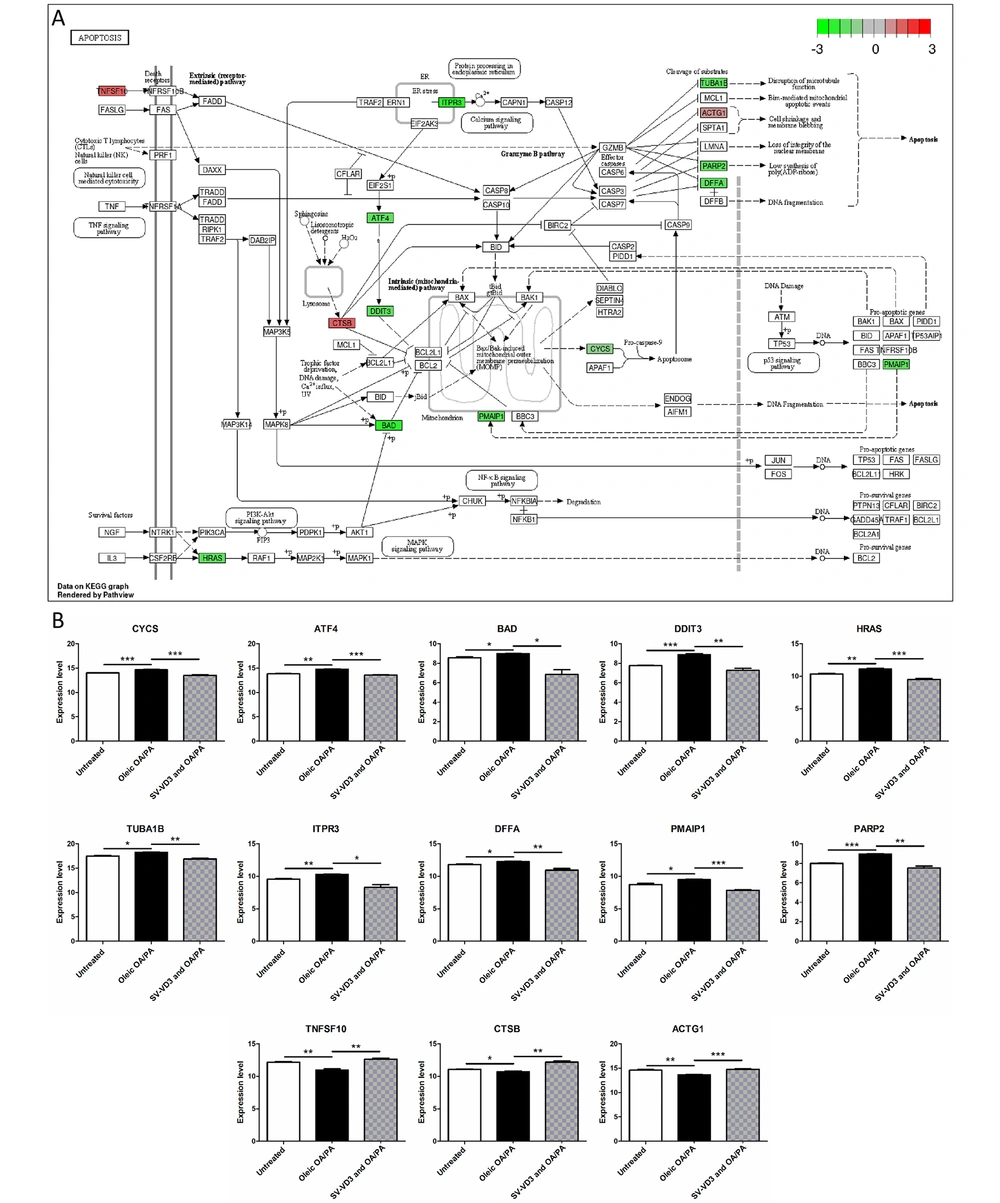
After SV-VD3 was added to HepaRG cells pre-treated with oleic acid OA/PA mixture, the expressions of DFFA (P = 0.0081), PARP2 (P = 0.0019), BAD (P = 0.0494), ITPR3 (P = 0.0441), TUBA1B (P = 0.0014), DDIT3 (P = 0.0028), PMAIP1 (P = 0.0001), CYCS (P = 0.0004), HRAS (P = 0.0010) and ATF4(P < 0.0001) were down-regulated. The expressions of ACTG1 (P = 0.0008), TNFSF10 (P = 0.0046) and CTSB (P = 0.0019) were up-regulated in the apoptosis pathway (Figure 6A and B). However, the expression of these genes was reversed before SV-VD3 was added (Figure 6B).
Vitamin D improves liver cell lipid toxicity by regulating the apoptosis pathway. (A) HepaRG cells treated with OA/PA and SV-VD3 cause gene expression changes in the apoptosis pathway. The image reference source is the KEGG Pathway database (16). The red gene represents up-regulation, while the green gene represents down-regulation. (B) The changing trend of apoptosis pathway genes under three treatments. *P < 0.05,**P < 0.01,***P < 0.001.
According to the analysis of differentially expressed genes in the NAFLD cell model, high-fat induction resulted in abnormalities in the cells' complement and coagulation cascades and cell cycle pathways. This mainly led to a decrease in complement and coagulation cascade functions and an increase in cell proliferation ability, which may be a potential mechanism for developing NAFLD. However, the addition of VD3 restored the completion and coalescence cascade pathway and cell cycle pathway and alleviated lipid-induced liver injury and fibrosis by reducing the cell apoptosis rate.
5. Discussion
A high-fat diet is an important factor in the development of NAFLD (17). As a pleiotropic hormone, vitamin D is involved in calcium homeostasis and contributes to the immune, inflammation, and metabolism (18). Some studies (19, 20) have confirmed that vitamin D deficiency is closely related to insulin resistance-related diseases, such as type 2 diabetes, NAFLD, and metabolic syndrome. Low vitamin D levels can be an independent factor in aggravating the degree of steatosis, tissue inflammation, and fibrosis in nonalcoholic fatty liver disease (21). In the study of Geier et al. (22), patients with NASH were supplemented with 2100 IU of vitamin D3 every day. After 48 weeks, the serum ALT levels of the patients with NASH were significantly reduced. This study aimed to explore the role of vitamin D in improving the lipotoxicity of hepatocytes by regulating the epigenetic characteristics of genes and to provide a new clue for the treatment of NAFLD.
Bioinformatics is based on gene chip research and obtains many complex biological information data from gene chips. It uses methods such as sequence alignment, statistical analysis, biological molecular network, pathway analysis, and visualization mapping to mine biological information data (23) to comprehensively and systematically study vitamin D regulation on the epigenetics of liver cells. This study screened gene expression differences and related mechanisms of HepaRG cells after oleic acid OA/PA and vitamin D3 treatment by analyzing the GSE200765 chip in the GEO database. After oleic acid OA/PA was added to HepaRG cells, 218 genes were up-regulated, and 255 were down-regulated. When SV-VD3 was added to HepaRG cells treated with OA/PA, 554 genes were up-regulated, and 704 were down-regulated.
The KEGG pathway analysis showed that the differentially expressed genes were enriched in the complement and coagulation cascades, cell cycle, and apoptosis pathways. The blood coagulation system is an important part of the body's immune response (24). Infection can simultaneously activate the blood coagulation system to induce blood coagulation, which will further limit the invasion of pathogens into the body (25). Complement and coagulation cascade systems help maintain the activation of natural immunity (26). NAFLD patients have primary coagulation abnormalities due to abnormal platelet aggregation and function. After the HepaRG cells were pre-treated with oleic acid OA/PA mixture, the expression of C4B, FGB, C4A, FGA, VTN, SERPING1, A2M, CLU, and C2 in the complex and coordination cascade pathways was down-regulated, and the expression of SERPINB2 was up-regulated. After SV-VD3 was added on this basis, the expression of the above genes was reversed. VD3 can partially restore the hepatocyte complex, and coagulation cascades pathway.
The cell cycle is divided into G1, S, G2, and M phases. Cell cycle transition is mainly regulated by cyclin-dependent kinase (CDK) and CDK inhibitor (CKI) (27). One study (28) found that a high-fat diet can promote the angiogenesis of the aorta, solid tumor growth, and tumor lung metastasis of BALB/C mice injected with CT26 colon cancer cells. Among them, the expression of CDK2 significantly increased in mouse tumor tissue, indicating that a high-fat diet can disrupt the cell cycle regulation mechanism, leading to unlimited cell proliferation and differentiation. This indicates that a high-fat diet can be important in cell cycle regulation and cancer progression. In this study, after the HepaRG cells were pre-treated with oleic acid OA/PA mixture, the expressions of PLK1, CDC6, CDC25C, ORC6, PTTG1, CDK1, SFN, and MAD2L1 were up-regulated, and TGFB1 was down-regulated in the cell cycle pathway. After SV-VD3 was added on this basis, the expression of the above genes was reversed. This shows that VD3 can reverse the disorder of the liver cell cycle to a certain extent.
In typical NAFLD patients (29), TUNEL methods and activated caspase immunohistochemical analysis have demonstrated that hepatocyte apoptosis is a significant pathological feature of human NAFLD, and the degree of hepatocyte apoptosis is positively correlated with AST/ALT ratio, inflammation degree, and fibrosis stage. Recently, studies on animal models in vivo and steatosis hepatocyte models in vitro have further proved that steatosis can induce hepatocyte death pathways and aggravate liver injury and fibrosis through the caspase six apoptotic protein (30). In this study, after SV-VD3 was added to HepaRG cells pre-treated with oleic acid OA/PA mixture, the expressions of DFFA, PARP2, BAD, ITPR3, TUBA1B, DDIT3, PMAIP1, CYCS, HRAS and ATF4 were down-regulated, and the expressions of ACTG1, TNFSF10 and CTBB were up-regulated in the apoptosis pathway. However, these apoptotic pathway genes' expression was reversed before SV-VD3 was added. This indicates that VD3 can partially reverse hepatocyte apoptosis induced by lipotoxicity.
To sum up, this study explores the dataset of vitamin D3's cytoprotective effect on human hepatocyte lipotoxicity through bioinformatics and analyzes its differentially expressed genes, key targets, and pathways. By adding this information to the existing literature, we illuminated the cytoprotective effect of vitamin D3 on human hepatocyte lipotoxicity. The inhibitory role of vitamin D3 in the lipotoxicity of human hepatocytes and the possible reversal mechanism were demonstrated by studying the gene expression trends of the pathways. These results demonstrated that vitamin D3 could improve liver cells’ lipotoxicity by regulating the genes on the complexity and coordination cascades, cell cycles, and apoptosis pathways in liver cells, which can pave the way for future treatment of NAFLD and liver-cell damage.