1. Background
The incidence of hepatocellular carcinoma (HCC) is continuously increasing, with > 700,000 new cases per year worldwide, mainly due to the booming incidence of chronic hepatitis C and B infections (1). To date, HCC is a significant contributor to cancer mortality, being the second biggest cause of cancer mortality in males and sixth in females worldwide (1). Although there is a substantial improvement in HCC diagnosis and treatment, the outcome remains poor, with a median survival time of six to 20 months and a five-year survival rate of only 6.9% (2).
Patients with HCC may associate chronic kidney disease (CKD) in 23% to 27% of cases (3, 4). Also, among patients with CKD, HCC represents one of the most common neoplastic diseases (5). In a large cohort of more than 35,000 pre-dialysis CKD patients, the standardized incidence ratio of HCC was 5.98, significantly higher than in the control cohort population (898,140 individuals) (6). The crosstalk between HCC and CKD is explained by the fact that HCC and CKD have common etiologies (7, 8). Chronic viral hepatitis B (CHB) and chronic viral hepatitis C (CHC) are recognized as the main risk factors for HCC, while Hepatitis B Virus (HBV) and hepatitis C virus (HCV) cause concomitantly chronic glomerulonephritis and CKD as extrahepatic complications, respectively (7). Moreover, liver cirrhosis (LC) increases the risk of Diabetes mellitus (DM), predisposing to the development of CKD in HCC patients (8). It is well established that there is an increased risk of cancer among patients with CKD and that patients with neoplasia have a higher incidence of CKD (9, 10).
Regarding the outcome of HCC in CKD populations, there are only a few contradictory studies (11, 12). Some authors demonstrated that HCC patients with CKD had a low survival rate (11, 13). On the other hand, in the study conducted by Huoo et al., survival rates were similar between HCC individuals with and without CKD (12). However, these data have predominantly considered renal function at baseline without evaluating the impact of dynamic modifications in the estimated glomerular filtration rate (eGFR) and the outcome of individuals with HCC.
Percutaneous ethanol injection therapy (PEIT), an ultrasound-guided percutaneous non-thermal ablation technique, was the most effective therapy for HCC patients considered poor surgical candidates for resection before the introduction of radiofrequency ablation (RFA) (14). It seems that RFA has a better outcome than PEIT (15). In contrast, several European studies reported similar survival rates for RFA and PEIT (16, 17). Furthermore, PEIT has minor complications, while serious adverse events, including hepatic infarction, hemothorax, and skin burns, may develop after RFA (14). In addition, PEIT is a valuable alternative where RFA is inaccessible (14). In our center, RFA was not available during the study.
2. Objectives
This study aimed to analyze the rate of decline in renal function, the factors linked to the rapid decline of renal function, and the relationship between rapid eGFR decline and mortality in HCC patients treated by PEIT.
3. Methods
3.1. Patients
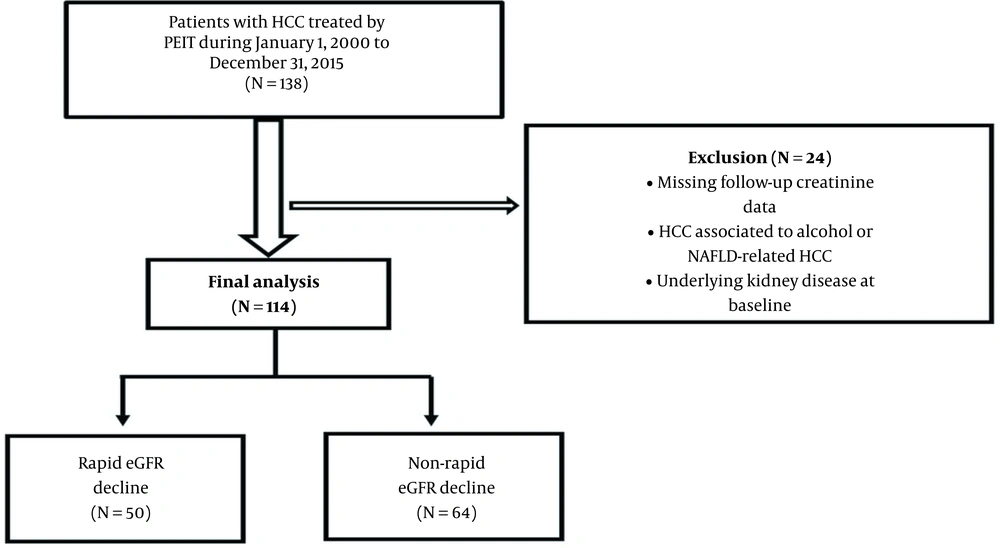
Our prospective observational study included 114 consecutive patients admitted from January 2000 through December 2015 to the Department of Gastroenterology and Hepatology Timisoara with HCC related to hepatotropic viruses such as HCV, HBV, and HBV/HCV coinfection treated with PEIT. The patients were followed-up until death or the end of the study (31 December 2015).
Demographics and comorbidities (coronary artery disease (CAD), DM, and prior stroke) were retrieved from the patient’s medical records. Initial laboratory results including coagulation tests, complete blood count, serum albumin, alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin, alkaline phosphatase (ALP), alpha-fetoprotein, and serum creatinine levels were also recorded.
3.2. Diagnosis of Hepatocarcinoma
The diagnosis of HCC was established by contrast CT or MRI and, in doubtful cases, by ultrasound-guided biopsy. The inclusion criteria were: One to three nodules with 2 - 5 cm diameter, thrombocyte counts > 50,000/mm3, prothrombin index > 50%, and no ascites. The severity of LC was assessed using the Child-Pugh criteria. For every patient, we calculated the cancer of the liver Italian program (CLIP) score for survival prediction according to CLIP investigators (18). Among patients undergoing non-surgical therapy, the CLIP score could better predict survival than the earlier versions of the scoring system as TNM, Okuda, or Barcelona clinic liver cancer classification (19).
3.3. Estimation of Renal Function and Outcome Measurements
Baseline eGFR was assessed using the creatinine-based chronic kidney disease epidemiology collaboration (CKD-EPI) equation (20). The baseline renal function in our cohort was considered the first eGFR measurement. The main outcome was to evaluate the accelerated decline of eGFR and its relationship with mortality in the studied patients. The accelerated decline of eGFR was defined as a variation of > 5 mL/min/1.73 m2 per year as stated by the kidney disease improving global outcomes (KDIGO 2012) guidelines (21). We used only the first and last eGFR measurements during the study period to calculate the eGFR decline rate. The absolute yearly eGFR decline rate was calculated as follows:
Subjects with less than two valid eGFR determinations were not included, as eGFR decline could not be estimated. Individuals with fewer than 90 days between the last and first eGFR determinations were also ruled out to minimize the effects of acute renal injury, attain more appropriate estimates of eGFR decline, and diminish the consequences of determination variability. Furthermore, we excluded the patients with HCC associated with alcohol consumption or non-alcoholic fatty liver disease-related HCC, the subjects with a history of chemotherapy and HIV infection, and those with underlying kidney disease at baseline.
3.4. Ethics Statement
All subjects gave informed consent for inclusion before participating in the study. The study was conducted following the declaration of Helsinki, and the Ethics Committee (Board of Human Studies) of the county emergency approved the protocol (approval number 1/3th January 2000).
3.5. Statistical Analysis
The data were analyzed using SPSS v.17 software (SPSS Inc. Chicago, IL, USA). Continuous variables with Gaussian distribution were expressed as mean ± standard deviations and those with non-Gaussian distribution as median (interquartile range). Percentages were used for categorical variables. For variables with Poisson distribution, the lower and upper limits of 95% confidence intervals (CIs), used to estimate the prevalence rates, were calculated according to Wilson’s procedure. Furthermore, the 95% CI for odds ratio (OR) was determined using the mid-p method for binomial distributions.
The student t-test (means, Gaussian populations), Mann-Whitney-U test (medians, non-Gaussian populations), chi-square test (proportions), and log-rank test (differences between survival curves and hazard ratio) assessed the significance of between-group differences. The Shapiro-Wilk test assessed the normal distribution of continuous variables, and Levene’s test determined the equality of variances. Logistic univariate regression analyses were performed to determine which parameters at baseline may predict the rapid decline of eGFR. Variables that showed statistical significance in the univariate analyses were included in the multivariable models. The multivariate Cox analysis evaluated the independent risk factors for rapid eGFR decline during the follow-up. We used Cox’s proportional hazards model to assess the association of rapid eGFR decline with mortality and estimate the hazard ratio and confidence interval. For all analyses, two-sided P < 0.05 was considered statistically significant.
4. Results
4.1. Patient Demographics and Prevalence of Rapid Kidney Function Decline
Our study included a cohort of 114 HCC patients treated by PEIT (Figure 1). Most patients were males (64.9 %), with a mean age of 65.17. In our cohort, 75.43% of HCC cases were attributed to HCV, 21.05% were associated with HBV, and 3.5% presented HBV/HCV coinfection (Table 1). During a median follow-up of 31 months, 43.85% of patients demonstrated a rapid decline in eGFR. We observed that hepatitis B infection was significantly more prevalent in the group without a rapid decline of eGFR (P = 0.04). Patients with a rapid decline of eGFR had a higher severity of LC than patients without a rapid decline of eGFR. Child-Pugh class B was found in 84% (n = 42) of patients in the group with rapid eGFR decline versus 0% of patients in the group with non-rapid eGFR decline (P < 0.0001). The CLIP score was significantly higher in HCC patients with a rapid decline of eGFR. Out of 50 patients with rapid kidney function decline, 16 (32%) patients had a CLIP score of 2 as compared to no patients (0%) with a non-rapid decline of eGFR (P < 0.001), while eight (16%) patients with the rapid decline of eGFR had a CLIP score of 3 versus no (0%) patients without rapid eGFR decline (P = 0.001) (Table 1). There was no difference in the number of PEIT sessions/patient in both groups (P = 0.68).
| Variables | Total Group (N = 114) | Rapid eGFR Decline (N = 50) | Non-rapid eGFR Decline (N = 64) | P-Value |
|---|---|---|---|---|
| Age (y) | 65.18 ± 8.55 | 64.3 ± 9.53 | 65.86 ± 7.71 | 0.33 |
| Male gender; No. (%) | 74 (64.91) | 29 (58) | 45 (70.31) | 0.23 |
| Etiology of LC; No. (%) | ||||
| HCV | 86 (75.43) | 42 (84) | 44 (68.75) | 0.07 |
| HBV | 24 (21.05) | 6 (12) | 18 (28.12) | 0.04 |
| HBV + HCV | 4 (3.5) | 2 (4) | 2 (3.12) | 0.80 |
| Child-Pugh class of LC; No. (%) | ||||
| Child-Pugh A | 72 (63.15) | 8 (16) | 64 (100) | < 0.001 |
| Child-Pugh B | 42 (36.84) | 42 (84) | 0 (0) | < 0.001 |
| CLIP score; No. (%) | ||||
| 0 | 28 (24.56) | 0 (0) | 28 (43.75) | < 0.001 |
| 1 | 56 (49.12) | 20 (40) | 36 (56.25) | 0.09 |
| 2 | 18(15.79 ) | 18 (36) | 0 (0) | < 0.001 |
| 3 | 12 (10.52) | 12 (24) | 0 (0) | < 0.001 |
| Comorbidities; No. (%) | ||||
| Coronary artery disease | 39 (34.21) | 29 (58) | 10 (15.62) | < 0.001 |
| Previous ischemic stroke | 13 (11.40) | 10 (20) | 3 (4.68) | 0.016 |
| Diabetes mellitus | 25 (21.92) | 21 (42) | 4 (6.25) | < 0.001 |
| Creatinine at baseline (mg/dL) | 0.83 (0.35) | 0.83 ± 0.23 | 0.91 (0.43) | 0.01 |
| eGFR at baseline (mL/min/1.73 m2) | 80.15 ± 23.59 | 86.08 ± 19.17 | 75.53 ± 25.74 | 0.013 |
| Creatinine at follow-up (mg/dL) | 1.12 (0.58) | 1.42 (0.8) | 0.91 (0.36) | < 0.001 |
| eGFR at follow-up (mL/min/1.73 m2) | 60.23 ± 27.97 | 43.16 (42.34) | 71.78 ± 24.38 | < 0.001 |
| AST (U/L) | 81 (52) | 89 (54) | 75.5 (50) | 0.79 |
| ALT (U/L) | 71 (58) | 72.5 (64) | 68 (56.3) | 0.48 |
| Bilirubin (mg/dL) | 1.26 (1) | 1.85 (1.3) | 0.79 ± 0.28 | < 0.001 |
| ALP (U/L) | 147 (106) | 167.5 ± 82.27 | 137 (67.8) | 0.14 |
| Albumin (g/dL) | 3.73 ± 0.62 | 3.2 ± 0.48 | 4.1 (0.4) | < 0.001 |
| Prothrombin time (s) | 13.28 ± 1.62 | 14.70 ± 1.21 | 12.17 ± 0.86 | < 0.001 |
| Platelet count (× 103/μL) | 92.7 (62.8) | 104.07 ± 42.35 | 88.0 (45.0) | 0.21 |
| Hemoglobin (g/dL) | 13.26 ± 1.74 | 12.03 ± 1.25 | 14.22 ± 1.44 | < 0.001 |
| PEIT sessions/patient | 3 (5) | 3 (5) | 3.5 (4.8) | 0.68 |
| Mortality; No. (%) | 51 (44.73 ) | 34 (68) | 17 (26.56) | < 0.001 |
| Duration of follow-up (mo) | 31 (15) | 33 (15.2) | 30 (15) | 0.13 |
Baseline Demographics, Laboratory, and Clinical Data of Patients in the Cohort
The comorbidities (CAD, previous ischemic stroke, and DM) were significantly higher in HCC patients with rapid eGFR decline. The hemoglobin level was significantly lower in patients with rapid eGFR decline than in those without rapid eGFR decline (P < 0.001). The baseline eGFR was significantly higher in the group with the rapid decline of kidney function: 86.08 ± 19.17 mL/min/1.73m2 vs. 75.53 ± 25.74 mL/min/1.73m2 (P = 0.001) (Table 1).
4.2. Association of Clinical and Laboratory Parameters with Rapid Kidney Function Decline
The univariate regression analysis evaluated the laboratory and clinical variables associated with rapid eGFR decline. Baseline eGFR (P = 0.017), total bilirubin (P < 0.001), serum albumin (P < 0.001), prothrombin time (P < 0.001), hemoglobin (P < 0.001), Child-Pugh score (P < 0.001), and CLIP score (P < 0.001) were correlated with the rapid decline of kidney function. There was a significant association between HCV infection (P = 0.03), DM (P < 0.001), CAD (P < 0.001), and previous stroke (P = 0.01) and the rapid decline in eGFR (Table 2).
| Parameters | Coef Beta | 95% CI | R2 | P-Value |
|---|---|---|---|---|
| eGFR at baseline (mL/min/1.73 m2) | 0.004 | 0.0008 to 0.008 | 0.05 | 0.017 |
| HBV infection | -0.202 | -0.41 to 0.009 | 0.03 | 0.06 |
| HCV infection | 0.234 | 0.01 to 0.46 | 0.04 | 0.03 |
| Total bilirubin (mg/dL) | 0.437 | 0.374 to 0.500 | 0.63 | < 0.001 |
| Serum albumin (g/dL) | -0.605 | -0.702 to -0.51 | 0.57 | < 0.001 |
| Prothrombin time (s) | 0.237 | 0.201 to 0.274 | 0.60 | < 0.001 |
| Hemoglobin (g/dL) | -0.18 | -0.22 to -0.14 | 0.39 | < 0.001 |
| Comorbidities | ||||
| Diabetes mellitus | 0.514 | 0.311 to 0.717 | 0.18 | < 0.001 |
| Coronary artery disease | 0.463 | 0.288 to 0.639 | 0.19 | < 0.001 |
| Previous stroke | 0.373 | 0.089 to 0.657 | 0.05 | 0.01 |
| Child-Pugh Score | 0.889 | 0.79 to 0.98 | 0.74 | < 0.001 |
| CLIP Score | 0.388 | 0.315 to 0.462 | 0.49 | < 0.001 |
Univariable Regression Analysis of Risk Factors for Rapid Kidney Function Decline
To evaluate the association of multiple factors with the risk of the rapid decline of kidney function in HCC patients treated by PEIT, a multiple, backward conditional (stepwise, acceptance threshold P < 0.1, exclusion threshold P > 0.2) Cox proportional hazards model was generated, adjusted for factors associated with the rapid decline of kidney function in univariate regression analysis. Total bilirubin, serum albumin, prothrombin time, and Child-Pugh score were not included as covariables since they are used to calculate the CLIP score per se. We found a significant influence on the risk of the rapid decline of kidney function for the CLIP score (HR = 2.55, 95% CI: 1.70 - 3.84, P < 0.001) (Table 3).
| Predictor | B | Wald | Hazard Ratio [95% CI] | P-Value |
|---|---|---|---|---|
| CLIP score | 0.94 | 20.50 | 2.55 [1.70 to 3.84] | < 0.001 a |
| Diabetes mellitus | 0.75 | 3.39 | 2.12 [0.95 to 4.72] | 0.06 |
| Coronary artery disease | -0.52 | 3.07 | 0.59 [0.33 to 1.06] | 0.08 |
Predictors for Rapid Decline of eGFR Accepted in the Cox Proportional-Hazards Model
4.3. Association of Rapid Kidney Function Decline with Mortality
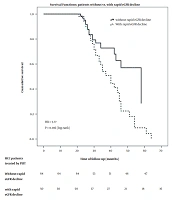
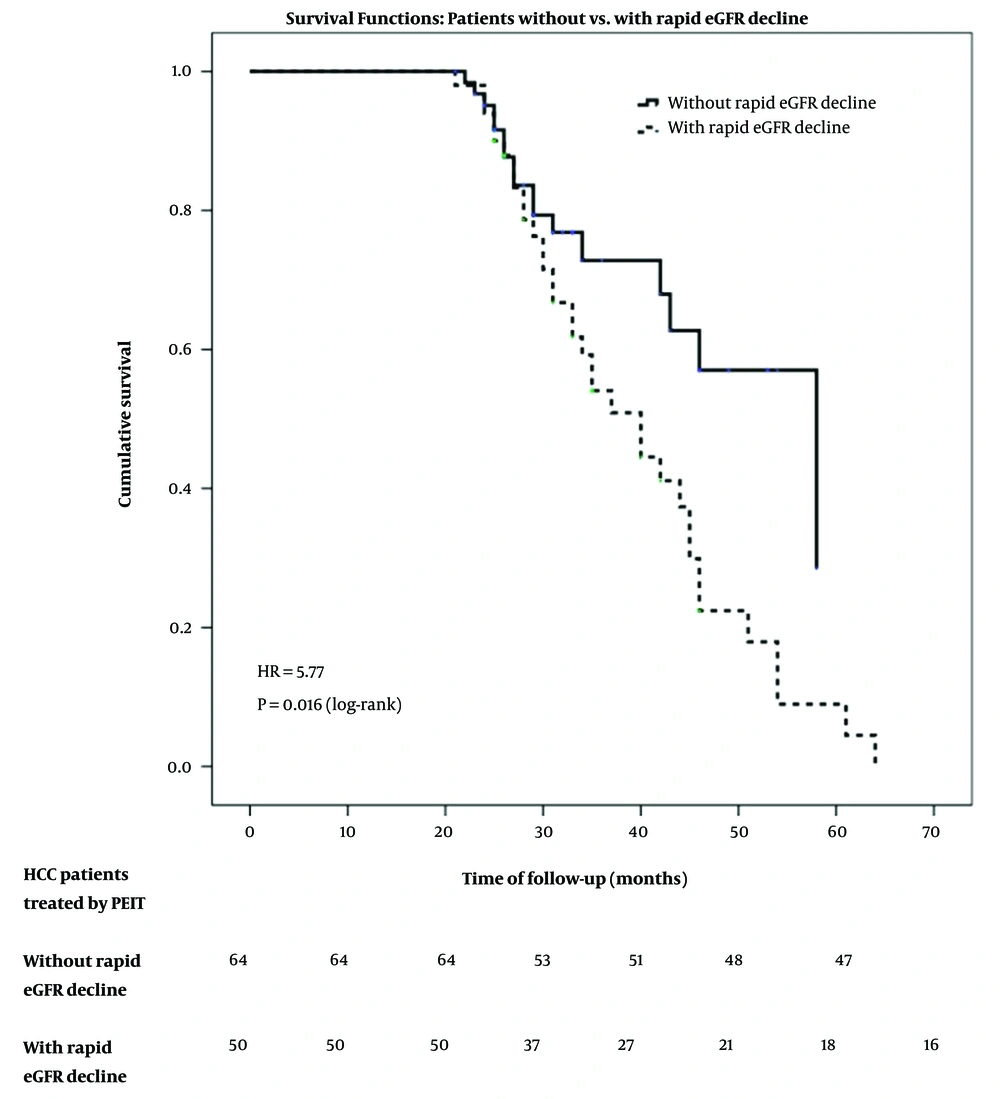
In our studied group, the overall mortality in the follow-up period was 44.73% (51 individuals), being higher in patients with a rapid decline of kidney function (68% vs. 26.56%, P < 0.001). The time-related prognosis was also influenced by the rapid decline of kidney function at the survival analysis resulting in an HR of 5.77 (P = 0.016) for mortality in HCC patients with rapid eGFR decline compared to those with non-rapid eGFR decline (Figure 2).
To assess the association of multiple factors with the risk of mortality, a multiple, backward conditional Cox proportional hazards model was generated, with the following co-factors: Rapid eGFR decline, CLIP score, hemoglobin, the presence of associated comorbidities (diabetes and stroke), infection with any of the two hepatic viruses, and the number of PEIT sessions. In the multivariate Cox regression model, the rapid decline of kidney function (dichotomous) (HR = 3.49, P = 0.015), CLIP score (HR = 4.37, P < 0.001), and the number of PEIT sessions (HR = 0.86, P < 0.001) were accepted as independent mortality predictors. The results of the obtained model are presented in Table 4.
Predictors for Mortality Accepted in the Cox Proportional-hazards Model
5. Discussion
The present study showed that rapid eGFR decline was common (43.85%) among HCC patients treated by PEIT. More rapid eGFR decline in HCC patients treated by PEIT was related to CLIP score severity. The rapid kidney function decline was an independent risk factor for HCC patients’ mortality, with an HR of 3.49 [95% CI: 1.28 - 9.56], P = 0.015.
Chronic kidney disease is one of the most common comorbidities in HCC patients, possibly because HCC and CKD share the same etiologic factors (3, 4, 22). However, rapid kidney function decline, defined as a sustained decline in eGFR of > 5 mL/min per 1.73 m2 per year (21), was not previously evaluated in HCC patients.
In this study, nearly one-half (43.85%) of HCC patients presented a rapid decline of eGFR compared to data from the general populations, revealing a rapid decline in kidney function in 16% of individuals (23).
In our paper, patients with rapid eGFR decline had a higher baseline eGFR than the non-rapid eGFR group. Other authors reported similar findings in both DM patients and general populations (24, 25). The relationship between higher baseline eGFR and rapid decline of renal function could be explained by glomerular structural damage due to persistent hemodynamic alterations such as vasodilatation in afferent arterioles and vasoconstriction in efferent arterioles (25, 26).
In the current study, CLIP score severity was a robust and independent predictor of the rapid decline of kidney function, with an HR of 2.55 (95% CI: 1.70 to 3.84; P < 0.001). As far as the current literature is concerned, there are no previous data regarding the CLIP score severity and risk of kidney function decline in HCC patients. The CLIP score, encompassing both tumor morphology and liver function, is considered one of the best prognostic staging systems in patients with HCC (27). The fact that the CLIP severity score is an independent risk factor for kidney function decline suggests the substantial crosstalk between the liver and kidney among HCC patients. Evidence suggests that the association of neoplasia with CKD leads to a worse outcome and complicates the management and treatment of patients (9).
Although the association between CKD and risk mortality in HCC patients has been explored in previous studies, the survival outcome in these patients remains controversial. Increasing evidence suggests that CKD as a comorbidity raises the mortality risk in HCC patients (4, 11, 13). Nonetheless, other authors have shown that CKD does not affect the survival of HCC patients (3, 12). Previous data have shown that in cancer patients, progressive reduction of renal function is linearly associated with increased mortality risk (28). In patients with HCC, Weng et al. demonstrated a graduated association between the severity of CKD and liver cancer mortality. Deaths from HCC increased progressively with the severity of renal impairment, with the adjusted HRs of mortality for individuals with an eGFR of 45 to 59, 30 to 44, 15 to 29, and < 15 mL/min per 1.73 m2 being 1.46, 2.99, 4.74, and 3.56, respectively, compared to patients with an eGFR above 60 mL/min per 1.73 m2 (P = 0.001 for trend) (22). Recently, another study revealed that higher serum creatinine levels in HCC patients were associated with a higher risk of early death (29).
The impact of rapid kidney function decline on mortality in patients with HCC was not evaluated previously. This study showed that rapid kidney function decline had a 3.49 (95%CI: 1.28 - 9.56) times increase in mortality risk in HCC patients treated by PEIT. These data are consistent with those obtained in the general population and patients with diabetes and/or hypertension (23, 30, 31). In the general population, Rifkin et al. found that individuals with a rapid decline of eGFR had a 1.7-fold increase in the risk of cardiovascular mortality (adjusted HR = 1.70; 95% CI: 1.40 - 2.06) and a 1.7-fold increase in the risk of all-cause mortality (HR = 1.73; 95% CI: 1.54 - 1.94) (23). Similar findings were revealed by Cheng et al. in a sizeable Taiwanese cohort of 17,026 participants (30). These authors showed that eGFR decline was associated with a 1.5-fold increase in the risk of death from all causes (adjusted HR = 1.45; 95% CI: 1.13 - 1.86) (30). Recently, another prospective analysis of a Chinese cohort demonstrated a significantly greater risk for all-cause mortality in individuals with kidney function decline (31).
The mechanisms by which reduced eGFR is related to the increased risk of cardiovascular disease and all-cause mortality are not fully understood. The possible explanation is that eGFR decline leads to accumulating inflammatory mediators, consequent augmentation of oxidative stress and endothelial dysfunction, and renin-angiotensin system activation (31). Moreover, renal function decline is associated with arterial hypertension and dyslipidemia, increasing the cardiovascular risk (23). Furthermore, in HCC patients, progressive reduction of renal function is associated with immunodeficiency, nutritional deviances, and possible retention of environmental carcinogens (mycotoxins, arsenic, and aristolochic acid), which play an essential role in the pathogenesis of HCC (9, 10, 22). Also, in females with HCC, CKD progression is associated with worsening hypogonadism and, thus, decreased protective effect of estrogens (3, 4, 32). In addition, decreased renal function was associated with appetite reduction, loss of muscle mass, and physical activity reduction, leading to a higher risk of mortality in these patients (22).
Besides, decreased kidney function in HCC patients could be a marker of hepatic disease progression, leading to augmented activation of the vasoconstrictor system and subsequent multiorgan hypoperfusion, including that of the kidneys, predisposing to a higher rate of mortality (3, 4, 11).
Our study has some limitations. The glomerular filtration rate estimates kidney function indirectly. Since direct calculations of GFR were not performed in this study, we could not evaluate if the eGFR variations appropriately reflected the actual modifications in renal function. Second, variables such as albuminuria or proteinuria were not assessed at baseline. Thus, we could not rule out the bias associated with some residual confounders. Third, the number of patients in this study was small, limiting the assessment accuracy of the associations.
Despite these limitations, this study has several strengths. First, as far as we know, this is the first study to delineate an association between the rapid decline of renal function and increased mortality risk in HCC patients. Second, this study showed an independent association between HCC severity evaluated by CLIP score and rapid decline of kidney function. Both of these findings underline the bidirectional damaging effects associated with liver-kidney crosstalk. Finally, our single center’s long-standing experience and our patients’ careful monitoring helped us highlight several essential conclusions.
5.1. Conclusions
This study showed the elevated prevalence of rapid decline in kidney function in HCC patients treated by PEIT. The relationship between rapid eGFR decline and increased mortality underlines the importance of early identification of HCC patients at risk for the rapid decline of kidney function.