1. Background
Among the malignant tumors with high morbidity and death rates, notably in Asia and Africa, is primary hepatocellular carcinoma (HCC) (1). According to reports, HCC is the second most frequent cause of cancer-related deaths in China and the sixth most frequent carcinoma (2, 3). Most cases of HCC in China are already in the intermediate and advanced stages at diagnosis, precluding traditional radical surgery as an effective intervention (4). For unresectable HCCs, transarterial chemoembolisation (TACE) is the most widely used treatment, playing an important role in prolonging patients’ survival and improving their quality of life (QoL) (3). It has been reported that over 100,000 patients with HCC undergo TACE in China every year (3).
The most frequent adverse event both during and after TACE is abdominal pain (5). Between 60% and 80% of patients report experiencing varying degrees of pain during and after TACE procedures (6, 7). Furthermore, 25% experience moderate to severe pain (6). Post-TACE pain imposes additional physical and psychological burdens on patients, reducing their compliance with treatment (8). Thus, suitable pain management is important to improve the QoL of patients with HCC undergoing TACE. Investigating the risk factors associated with post-TACE pain will help predict its occurrence and facilitate the establishment of a pain control plan to reduce patients’ pain.
Although some studies have analyzed the factors associated with post-TACE pain, no definite conclusions have yet been drawn. In one study, Bian et al. (3) analyzed data from 522 patients who underwent a total of 582 TACE operations and discovered that blood vessel invasion, TACE with drug-eluting beads (DEB-TACE), a history of abdominal discomfort after TACE, and a history of TACE were the predictors of acute moderate to severe pain (3). Pachev et al. (9) studied 80 patients with HCC and identified that age, cirrhosis, and alcoholic liver disease were negative predictive factors for severe abdominal pain.
2. Objectives
We conducted this prospective observational single-centre study to investigate the potential risk factors associated with post-TACE pain in patients with HCC and to create and verify a prediction model for predicting moderate to severe post-TACE discomfort.
3. Methods
3.1. Study Design
The ethics committee of our institution approved this prospective observational single-centre research, and our protocol conformed with the principles of the Declaration of Helsinki. Before participating in the study, each subject provided written permission. We included all patients with HCC undergoing TACE in our hospital from September 2019 to January 2021. According to this period, the dataset was divided into development and validation cohorts in a 3: 2 ratio. The diagnosis of HCC was confirmed either histologically or based on reliable results from at least two imaging procedures. The main exclusion criteria were a patient age of < 18 years, significant heart or lung malfunction, the extra use of analgesics to alleviate pain during TACE, cognitive impairment, the use of psychiatric drugs, and drug/alcohol abuse. This study was registered at www.clinicaltrials.gov (registry number: NCT05545046).
Indications for TACE were in accordance with the Chinese clinical practice guidelines for conducting TACE in patients with HCC (10). The indications were as follows: (1) Child-Pugh liver function class A or B; (2) an expected survival time of > 3 months; (3) an Eastern Cooperative Oncology Group (ECOG) score of 0 - 2; (4) liver cancer at clinical stage IIb or IIIa (or patients with stage Ib or IIa who could be managed surgically but were contraindicated or unwilling to undergo surgery or local ablation due to other reasons, such as advanced age and severe cirrhosis, as well as some patients with stage IIIb with extrahepatic metastases who were expected to benefit from TACE to control the growth of intrahepatic tumors). Transarterial chemoembolisation is indicated for patients with large liver tumors, where the tumor accounts for 70% of the entire liver, for patients with HCC whose main portal vein is not completely blocked, for those whose portal compensatory collateral vessels are abundant despite complete obstruction or whose portal vein blood flow can be restored by portal vein stent placement and for patients suffering from portal hypertension caused by the rupture of liver cancer and a hepatic arterioportal shunt. Prophylactic TACE can be a choice for patients with HCC with risk factors such as multiple tumors, combined macroscopic or microscopic tumor thrombectomy, a history of palliative surgery, and persistently high levels of tumor markers (such as AFP) even after surgery. This procedure should be used for the early detection and treatment of residual or relapsed cancer after resection or liver transplantation and for tumor reduction therapy before HCC surgery to reduce the tumor stage and create opportunities for stage-II resection or liver transplantation.
3.2. DEB-TACE Procedure
All the research participants underwent percutaneous femoral artery puncture after receiving a local anaesthetic of 10 mL of 2% lidocaine. First, the hepatic artery was catheterised, followed by the femoral artery, using an arterial catheter. Then, either a standard TACE procedure or a DEB-TACE procedure was performed. Patients did not receive both DEBs and lipiodol when they underwent DEB-TACE. All patients received supportive care after TACE, including antiemetics, liver protection, and NSAIDs.
During their preoperative visit, the patients were taught how to use a visual analog scale to correctly ascertain their pain on a linear scale from 0 to 10 within 24 hours of embolisation; a score of 0 represented no pain, 3 represented mild pain, 4 to 6 represented moderate pain, and scores between 7 and 10 reflected severe pain. The patients declared their pain intensity at rest and 0, 2, 4, 6, 12, and 24 hours after TACE. The highest score recorded for a patient throughout the period was their final score.
3.3. Data Collection
The data collected included the patient’s age, gender, ECOG score, history of chronic hepatitis, tumor location, size and number of tumors, drug delivery method (traditional TACE vs. DEB-TACE), dose of lipiodol, Child-Pugh class, history of postembolisation syndrome, T stage, Barcelona Clinic Liver Cancer (BCLC) staging class and the presence of portal vein tumor thrombosis (PVTT) prior to TACE. Standard computed tomography served as the basis for the diagnosis of PVTT.
3.4. Statistical Analysis
The data were analyzed using SPSS (version 22.0) and MedCalc (version 19.0.5) statistical tools. Categorical data were expressed as percentages and numbers, and quantitative data were described using the mean and standard deviation. Patient characteristics were compared using the Chi-squared test or Fisher’s exact test for categorical data and a t test or one-way ANOVA for continuous data.
A multivariate logistic regression model was created to identify the independent determinants of post-TACE pain. The risk score or weight index of each variable was determined by the odds ratio in the logistic equation and used to simplify the calculation. The total risk score was regarded as the sum of the scores of the variables multiplied by the coefficients plus the intercepts. The area under the receiver operating characteristic (ROC) curve (AUC), the specificity, and the sensitivity were obtained using a ROC curve analysis to assess the performance of the prediction model. Statistical significance was defined as a two-tailed P value of < 0.05. To date, there is no consensus regarding sample size calculation for studies that develop or validate prediction models (11). In this study, the number of participants presenting the outcome respective to the number of potential predictors was consistent with the recommendations of the Prediction Model Risk-of-Bias Assessment Tool (12).
4. Results
From September 2019 to January 2021, 228 patients with HCC who underwent TACE were enrolled in the study, including 137 patients in the development cohort (mean age: 60.3 ± 10.1 years; 78.1% male) and 91 patients in the validation cohort (mean age: 61.1 ± 10.5 years; 73.6% male). A total of 196 (85.96%) patients underwent traditional TACE, and 32 (14.04%) underwent DEB-TACE. The overall median pain score of all the patients was 2.0 (2.0, 7.0). Furthermore, 46.0% and 39.6% of the patients in the development and validation cohorts experienced acute moderate to severe post-TACE pain, respectively. The only clinical parameter that significantly differed between the two groups was only the proportion of stage-II tumors (32.8% vs. 49.5%, P = 0.012). Table 1 presents the characteristics of the patients in the two groups.
| Variables | Development Cohort (n = 137) | Validation Cohort (n = 91) | P-Value |
|---|---|---|---|
| Age (y) | 60.3 ± 10.1 | 61.1 ± 10.5 | 0.592 |
| Male | 107 (78.1) | 67 (73.6) | 0.436 |
| ECOG score | 0.549 | ||
| 0 - 1 | 131 (95.6) | 85 (93.4) | |
| 2 | 6 (4.4) | 6 (6.6) | |
| History of chronic hepatitis | 126 (92.0) | 82 (90.1) | 0.627 |
| History of postembolisation syndrome | 86 (62.8) | 62 (68.1) | 0.406 |
| Child-Pugh class | 0.689 | ||
| A | 96 (70.1) | 66 (72.5) | |
| B | 41 (29.9) | 25 (27.5) | |
| Tumor location (> 1 cm away from hepatic capsular) | 33 (24.1) | 19 (20.9) | 0.572 |
| Tumor size (≥ 5 cm) | 69 (50.4) | 45 (49.5) | 0.892 |
| Tumor number (≥ 2) | 45 (32.8) | 45 (49.5) | 0.012 |
| T stage | 0.947 | ||
| I | 40 (29.2) | 28 (30.8) | |
| II | 54 (39.4) | 34 (37.4) | |
| III | 43 (31.4) | 29 (31.9) | |
| Dosage of lipiodol (≥ 10 mL) | 7 (5.1) | 7 (7.7) | 0.426 |
| DEB-TACE | 22 (16.1) | 10 (11.0) | 0.281 |
| BCLC staging classification | 0.771 | ||
| A | 35 (25.5) | 27 (29.7) | |
| B | 56 (40.9) | 34 (37.4) | |
| C | 46 (33.6) | 30 (33.0) | |
| PVTT | 40 (29.2) | 26 (28.6) | 0.919 |
| Moderate to severe pain | 63 (46.0) | 36 (39.6) |
Clinical Characteristics of the Study Population a
In the development cohort, there were 63 and 74 patients with and without post-TACE pain, respectively. Compared with patients without moderate to severe pain, those with moderate to severe pain showed a higher proportion of tumors located ≤ 1 cm away from the hepatic capsular (P = 0.038), tumor size ≥ 5 cm (P = 0.032), T III stage (P = 0.002), DEB-TACE (P = 0.023), BCLC staging (P = 0.004) and PVTT (P = 0.001). Patients with post-TACE pain were younger than those without post-TACE pain (58.5 ± 9.8 years vs. 61.9 ± 10.1 years, P = 0.053), but the difference was not statistically significant (Table 2).
| Variables | No Post-TACE Pain (n = 74) | Post-TACE Pain (n = 63) | P-Value |
|---|---|---|---|
| Age (y) | 61.9 ± 10.1 | 58.5 ± 9.8 | 0.053 |
| Male | 56 (75.7) | 51 (81.0) | 0.457 |
| ECOG score | 0.413 | ||
| 0 - 1 | 72 (97.3) | 59 (93.7) | |
| 2 | 2 (2.7) | 4 (6.3) | |
| History of chronic hepatitis | 68 (91.9) | 58 (92.1) | 0.971 |
| History of postembolisation syndrome | 48 (64.9) | 38 (60.3) | 0.583 |
| Child-Pugh class | 0.422 | ||
| A | 54 (73.0) | 42 (66.7) | |
| B | 20 (27.0) | 21 (33.3) | |
| Tumor location (> 1 cm away from hepatic capsular) | 23 (31.1) | 10 (15.9) | 0.038 |
| Tumor size (≥ 5 cm) | 31 (41.9) | 38 (60.3) | 0.032 |
| Tumor number (≥ 2) | 26 (35.1) | 19 (30.2) | 0.537 |
| T stage | 0.002 | ||
| I | 27 (36.5) | 13 (20.6) | |
| II | 33 (44.6) | 21 (33.3) | |
| III | 14 (18.9) | 29 (46.0) | |
| Dosage of lipiodol (≥ 10 mL) | 3 (4.1) | 4 (6.3) | 0.703 |
| DEB-TACE | 7 (9.5) | 15 (23.8) | 0.023 |
| BCLC staging classification | 0.004 | ||
| A | 24 (32.4) | 11 (17.5) | |
| B | 34 (45.9) | 22 (34.9) | |
| C | 16 (21.6) | 30 (47.6) | |
| PVTT | 13 (17.6) | 27 (42.9) | 0.001 |
Comparison of Patients’ Clinical Characteristics According to the Presence or Absence of Post-transarterial Chemoembolisation Moderate-to-Severe Pain in the Development Cohort a
After adjusting for age, tumor size, T stage, and BCLC class, multivariate logistic regression showed that the tumor location, the drug delivery method, and the presence of PVTT were independently associated with post-TACE pain (Table 3). Subsequently, we developed the following risk score equation using a combination of these factors:
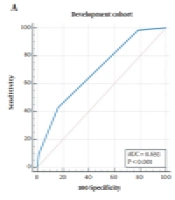
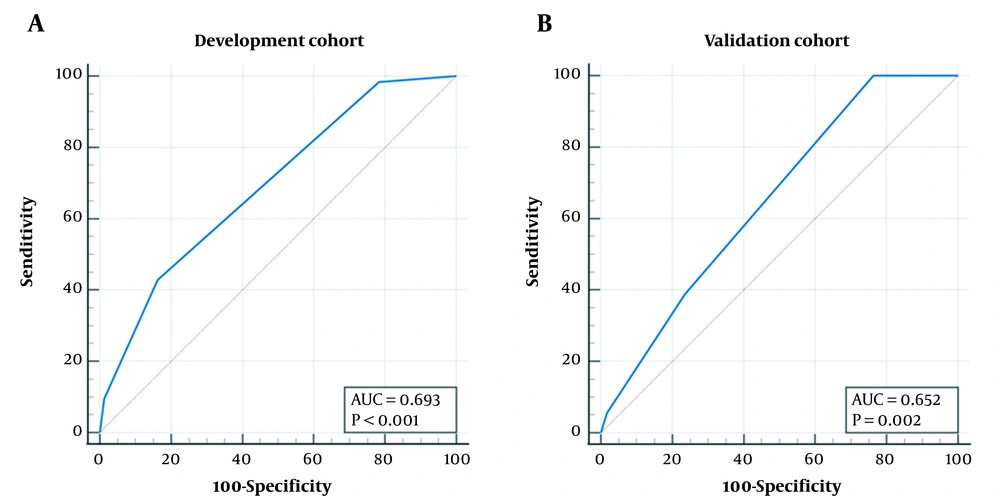
Risk score = -0.5 - 1 × tumor location > 1 cm away from the hepatic capsular (‘yes’ = 1, ‘no’ = 0) + 1 × DEB-TACE (‘yes’ = 1, ‘no’ = 0) + 1 × PVTT (‘yes’ = 1, ‘no’ = 0). In the development cohort, the AUC was 0.693 (95% confidence interval (CI): 0.609 to 0.769, P < 0.001), and the best cut-off value was > -0.5, with a sensitivity of 42.9% (95% CI: 30.5% to 56.0%) and a specificity of 83.8% (95% CI: 73.4% to 91.3%) (Figure 1A). In the validation cohort, the AUC was 0.652 (95% CI: 0.544 to 0.748, P = 0.002), and at the cut-off value of > -0.5, the sensitivity was 38.9% (95% CI: 23.1% to 56.5%), with a specificity of 76.4% (95% CI: 63.0% to 86.8%) (Figure 1B). There was no significant difference between the two cohorts (difference: 0.042, 95% CI: -0.081 to 0.164, P = 0.506).
| Variables | Regression Coefficient | OR | 95% CI for OR | P-Value |
|---|---|---|---|---|
| Intercept | -0.462 | - | - | 0.053 |
| Age | - | - | - | - |
| Tumor location (> 1 cm away from hepatic capsular) | -1.172 | 0.310 | 0.122 to 0.784 | 0.013 |
| Tumor size (≥ 5 cm) | - | - | - | - |
| T stage | - | - | - | - |
| DEB-TACE | 1.278 | 3.588 | 1.210 to 10.640 | 0.021 |
| BCLC staging classification | - | - | - | - |
| PVTT | 1.290 | 3.632 | 1.592 to 8.288 | 0.002 |
Multivariate Analysis of the Factors Related to Post-transarterial Chemoembolisation Pain in the Development Cohort
5. Discussion
We investigated the risk factors associated with post-TACE pain in patients with HCC and combined these risk factors to develop a clinical prediction model. In the present study, acute moderate to severe pain after TACE was present in 43.86% (100/228) of the patients. We found that the tumor location, the drug delivery method, and the presence of PVTT were significantly associated with post-TACE pain. Using these factors, we developed a prediction model to calculate a risk score, which was useful in both the development and validation cohorts. Although some predictors have been reported in previous studies, we validated the association of these predictors with post-TACE pain in a Chinese population. In addition, we constructed a novel prediction model by combining these independent predictors, which provided a simple and useful tool for clinical decision-making.
Pain after TACE is a common symptom of postembolisation syndrome. According to reports, between 60% and 80% of patients experience varying degrees of post-TACE pain (7, 13). Post-TACE pain has been reported to increase the likelihood of hospitalization and decrease QoL in patients with HCC (14). The cause of post-TACE pain is not fully understood. Tumor hypoxia and necrosis, inflammatory responses, and cytokine release have been proposed as possible causes of post-TACE pain (15, 16). The identification of preoperative predictors of post-TACE pain is valuable but challenging.
After systematically screening and reviewing 28 relevant articles on post-TACE pain, we identified several independent predictors reported in four of these studies (3, 9, 10, 17) (Table 4). Some of these predictors were found to be non-significant in our study, which may have been due to differences in ethnicity and study design. Interestingly, we identified tumor location as a new predictor for post-TACE pain and developed a predictive score for estimating such risk in patients with HCC, thereby providing further insights into this field.
| Variables | Indicators for a Higher Probability |
|---|---|
| A history of abdominal pain after TACE | Yes |
| Operation method | Drug-loaded microspheres TACE |
| No. of TACE | < 3 times |
| No. of tumors | ≥ 3 |
| Tumor size | Diameter > 5 cm |
| Age | < 60 years old |
| Vessel infiltration | Yes |
| Cirrhosis | No |
| Alcoholic liver disease | No |
Independent Predictors of Post-transarterial Chemoembolisation Pain in Previous Studies
According to a study by Bian et al. (3), blood vessel invasion, a history of TACE, undergoing DEB-TACE, and a history of abdominal pain after TACE were the key predictors of acute moderate to severe pain after TACE. Furthermore, Pachev et al. (9) identified age, cirrhosis, and alcoholic liver disease as the negative predictors of severe abdominal pain. In another study that investigated the risk factors for discomfort after DEB-TACE in patients with HCC, the Branch of Interventional Physicians, Chinese Medical Doctor Association (10) reported PVTT and non-super-selective chemoembolisation as independent risk factors. Additionally, Benzakoun et al. (17) discovered that young patients without chronic liver disease were more prone to experiencing excruciating pain following TACE. In the present study, we also established a predictive model to determine the risk factors for post-TACE pain. The aforementioned studies also noted DEB-TACE and PVTT as risk factors for pain after TACE, which aligns with our observation.
Age is a common risk factor for pain after TACE. Pachev et al. (9) and Benzakoun et al. (17) demonstrated that younger patients were more likely to experience severe pain after TACE. The relationship between age and pain is complicated. Older patients have been reported to be more fragile and possibly more susceptible to pain (17). Herein, we also demonstrated that older patients (≥ 60 years) tended to have lower pain scores compared with younger patients.
In conventional TACE, chemotherapeutics are loaded onto lipiodol to embolise blood arteries and destroy tumor cells, while DEB-TACE, a variant of TACE, precisely delivers chemotherapeutic agents and controls their release through drug-loaded microspheres (18). Previously, DEB-TACE was shown to lower the incidence of adverse events, delivering efficacy equal to that of traditional TACE (19). However, it remains controversial as to whether DEB-TACE increases the likelihood of abdominal pain (20). Golfieri et al. (21) found that DEB-TACE had an advantage over traditional TACE in terms of lowering the incidence of postembolisation pain. In contrast, Bian et al. (3) found that patients undergoing DEB-TACE experienced more postembolisation pain than those undergoing TACE alone. Research by Khalaf et al. (22) and Baur et al. (23) also revealed that patients managed with DEB-TACE more frequently experienced severe pain than those receiving conventional TACE. Our results supported the conclusion that patients undergoing DEB-TACE have higher pain scores. Thus, DEB-TACE can be regarded as a risk factor for post-TACE pain.
In line with previous report (10), the current investigation also identified PVTT as a risk factor for post-TACE discomfort. The portal vein and hepatic artery supply blood to the liver independently. The blood flow of the hepatic artery increases in patients with PVTT to compensate for the portal vein’s lack of blood flow. Consequently, the embolisation of the hepatic artery in patients with PVTT will worsen hepatic ischemia and increase discomfort (13).
Our findings also suggested that the location of the tumor was significantly associated with post-TACE pain. In the present study, patients with a tumor located ≤ 1 cm away from the hepatic capsular had higher pain scores than those with a tumor located > 1 cm away. We hypothesized that the embolisation of tumors adjacent to the hepatic capsular might promote the stretching of the hepatic capsule, resulting in severe pain. However, post-TACE pain was not used to account for this factor (13, 24). It has been suggested that resected tumors measuring > 5 cm increase the risk of postembolisation syndrome after TACE (25). In our study, due to the small sample size, we excluded tumors measuring ≥ 5 cm from the multivariable analysis.
Based on the risk factors identified, we built a predictive model for post-TACE pain. We demonstrated that the predictive model had acceptable discriminative power and good specificity. More comprehensive analgesic interventions, such as multimodal analgesic therapy, are recommended for patients susceptible to pain (6). Using our predictive model, we could estimate the risk of post-TACE pain before surgery, enabling the implementation of an appropriate pain management plan for patients with different pain risks according to the predictive model.
The current research has several limitations. First, this study involved only one center, and external validation was not available for this research due to its relatively small sample size. Therefore, high-quality external validation is required to verify the validity and generalisability of this prediction model in different populations. Second, some risk factors, which were demonstrated to contribute to postembolisation pain in previous studies, such as chronic liver disease and psychological factors, were not investigated in this research (3, 9). Thus, future studies should develop a more robust prediction model that includes more potential contributing risk factors. Finally, we did not confirm whether our predictive model could provide information for effective pain management in patients with HCC after TACE. Therefore, future studies should be conducted to further determine the value of our prediction model.
5.1. Conclusions
In conclusion, our results showed that tumor location, the drug delivery method, and the presence of PVTT were significantly and independently associated with post-TACE pain. The prediction model developed based on these risk factors was useful in identifying patients at risk of moderate to severe pain after TACE. More research is required to ascertain the utility of our prediction model to enhance the effectiveness of pain management after TACE.