1. Background
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumor and the third cause of tumor-related mortality worldwide (1). Hepatocellular carcinoma is particularly prevalent in China, with 466 000 new cases and 422 000 deaths annually, accounting for 55.4% of the incidence and 53.9% of mortality in the world, according to the Chinese Society of Clinical Oncology (CSCO). Surgical resection, transarterial chemoembolization (TACE), microwave ablation, and liver transplantation were the common invasive treatment for HCC (2). However, the vast majority of HCC patients are diagnosed at an advanced stage, losing the opportunity of noninvasive therapy. The multitargeted small molecule tyrosine kinase inhibitor (TKI) of sorafenib or lenvatinib was the first-line treatment for unresectable HCC with unsatisfactory efficiency (3, 4). Apatinib is a new oral angiogenesis inhibitor targeting intracellular ATP binding sites of VEGFR-2, used as the second-line treatment for HCC (5). Recent studies have also shown that apatinib monotherapy as a first-line treatment is effective in patients with advanced HCC (6-9). Moreover, apatinib combined with immunotherapy showed robust efficacy and controllable safety in both first-line and second-line treatments for advanced HCC in a phase II clinical trial (10).
In humans, small regulatory RNAs play an important role in many momentous biological pathways, including development, guiding growth, differentiation, and apoptosis (11, 12). While the overwhelming majority of small regulatory RNAs are microRNA (miRNA). The involvement of miRNA in HCC tumorigenesis by participating in the inflammatory response and angiogenesis has been increasingly elucidated (13-17). According to the literature, miRNAs such as miRNA-18a, miRNA-630, miR-502, and miRNA-210 have been identified to be associated with the growth, migration, and invasion of HCC (18-21). Dicer is a key enzyme in the miRNA maturation process that plays an essential role in chromatin structure remodeling, inflammation, and apoptotic DNA degradation (22-24). Multiple studies have confirmed that Dicer expression is associated with the inhibition of carcinoma progression, such as colitis-associated tumorigenesis, human clear cell renal cell carcinoma, ovarian cancer, and breast cancer (16, 19, 25). Our previous study found that Dicer inhibited HCC by inhibiting proliferation, invasion, and migration (26, 27), but the downstream pathway remains to be further clarified.
Inflammation is closely related to tumor development (28-33). As a member of the inflammation cytokine, interleukin 6 (IL-6) involves not only in response to injury or infection but also in immune diseases and cancer (34). Interleukin 6 has been found to participate in a series of processes to modify tumor development, including tumorigenicity, anti-apoptosis, angiogenesis, proliferation, invasion, metastasis, and drug resistance (29, 35-38). Interleukin 6 overexpressed in HCC tissue could promote the occurrence and development of HCC (39-41). Apatinib enhances the efficacy of anti-PD-1 immunotherapy on HCC by inhibiting IL-6 secretion (42).
2. Objectives
This study investigated whether Dicer regulates HCC via the IL-6 pathway.
3. Methods
3.1. Cells, Cell Culture, and Reagents
Hepatic carcinoma cell line SMMC-7721 (26) was provided by the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were detected using the mycoplasma test, authenticated by short tandem repeat analysis, and cultured at a constant temperature of 37°C in the Dulbecco Modified Eagle Medium (DMEM), high-glucose medium (Gibco Life Technologies, Grand Island, NY), complemented with 10% fetal bovine serum (FBS; Gibco Life Technologies, Grand Island, NY) in a humidified incubator containing 5% CO2. Recombinant human IL-6 (RhIL-6; Meilun Biotechnology Co, Ltd, Dalian, China) was separated and stored in a refrigerator at -20°C. Tocilizumab was purchased from Chugai Pharma Manufacturing Company (Basel, Switzerland). Apatinib mesylate was purchased from Sigma‐Aldrich (St Louis, MO, USA).
3.2. Transfection
The SMMC-7721 cell transfected with a green fluorescent protein (GFP)-tagged Dicer-overexpressing lentivirus (pCMV-Dicer) or negative control lentivirus (pCMV-NC) was used for the following functional assay. The procedure of transfection and verification (the successful transfection of Dicer) has been described previously (26).
3.3. Flow Cytometry/Immunomagnetic Bead Method
The cells were cultured for 72 hours; then, the conditioned medium was collected, and after centrifugation, the supernatant was used to detect IL-6 using the flow cytometry/immunomagnetic bead method with LEGEND Plex Human Thrombosis Panel (3-plex) w/FP (BioLegend, USA) according to the manufacturer instructions strictly.
3.4. Cell Proliferation Assay
The cell proliferation assay was measured by cell counting kit-8 (CCK-8, DoJindo Lab, Kumamoto, Japan). Briefly, the cells (2 × 103) in the serum‐containing medium were seeded into 96-well microplates with 6 duplicate wells for each group. The cells were treated with RhIL-6, tocilizumab, and apatinib for 72 or 96 hours. At each time point (0, 24, 48, 72, and 96 hours), 10 µL of CCK-8 was added to each well and then incubated in a 5% CO2 humidified incubator for 2 hours at 37°C. Absorbance was measured at 450 nm using a microplate auto reader (BioTek Instruments, Inc, USA).
3.5. Cell Migration Assays
Cells were seeded on 6-well plates, and when the cell confluence reached approximately 100%, two straight lines were scratched on the surface of monolayer cells by a tip of a 200-µL pipette. Then, they were gently washed twice with PBS and replaced with serum-free culture media. Images were taken by a microscope 0 and 24 hours after the scratch. At least 5 fields were analyzed for each scratch, and the cell mobility was calculated using the following formula:
3.6. Cell Invasion Assays
The cell invasion assay was performed using 8-μm span transwell chambers (aperture 8; Corning, NY, USA) precoated with 1 mg/mL Matrigel (BD Biosciences, NJ)". The pCMV-Dicer or pCMV-NC of stably transfected cells (2 × 104 cells per well) were cultured in the upper chamber in 200-μL serum-free medium containing different drugs (RhIL-6, tocilizumab, or apatinib). Then, 500-μL DMEM medium containing 15% FBS was added to the lower chamber at 37°C for 24 hours. Next, the removed cells on the Matrigel membrane surface leave the cells that invaded the underside of the membrane. The remaining cells were fixed with 4% paraformaldehyde for 20 minutes and stained with 0.1% crystal violet for 30 minutes. The stained cells were counted under an inverted microscope (Nikon, Tokyo, Japan) with 5 randomly selected domains (magnification × 200).
3.7. Statistical Analysis
Statistics analyses were performed using SPSS version 21 (SPSS Inc, Chicago, IL, USA). One-way analysis of variance (ANOVA) was used to compare groups. Differences between the 2 groups were performed using the student t test. P values less than 0.05 were considered statistically significant.
4. Results
4.1. Dicer Inhibits HCC by the IL-6 Pathway
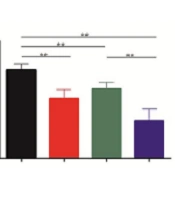
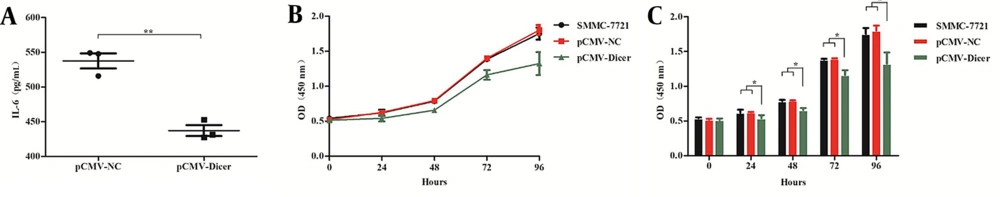
We measured the level of IL-6 in the medium of both pCMV-Dicer- and pCMV-NC–transfected HCC cells. As shown in Figure 1A, the IL-6 level of the pCMV-Dicer group was significantly downregulated compared to that pCMV-NC group (P = 0.002). These results indicated that Dicer overexpression could reduce IL-6 expression in HCC cells.
Dicer suppresses the interleukin 6 secretion and the proliferation of the hepatocellular carcinoma cell of 7721. A, interleukin 6 (IL-6) levels secreted from pCMV-NC and pCMV-Dicer cells; B, the proliferation curve measured by the cell counting kit-8 (CCK-8) assay for 7721, pCMV-NC, and pCMV-Dicer cells; C, the error bar of the CCK-8 assay of B. * P < 0.05, ** P < 0.01.
We detected the inhibitory effect of Dicer on HCC cells, and the results showed that compared with SMMC-7721 cells and pCMV-NC cells, the proliferation of pCMV-Dicer cells significantly decreased when cultured from 24 to 96 hours (Figure 1B and C; P < 0.05). Also, the migration (Figure 2C and D; P = 0.002) and invasion (Figure 2E and F; P = 0.034) of the pCMV-Dicer group significantly decreased.
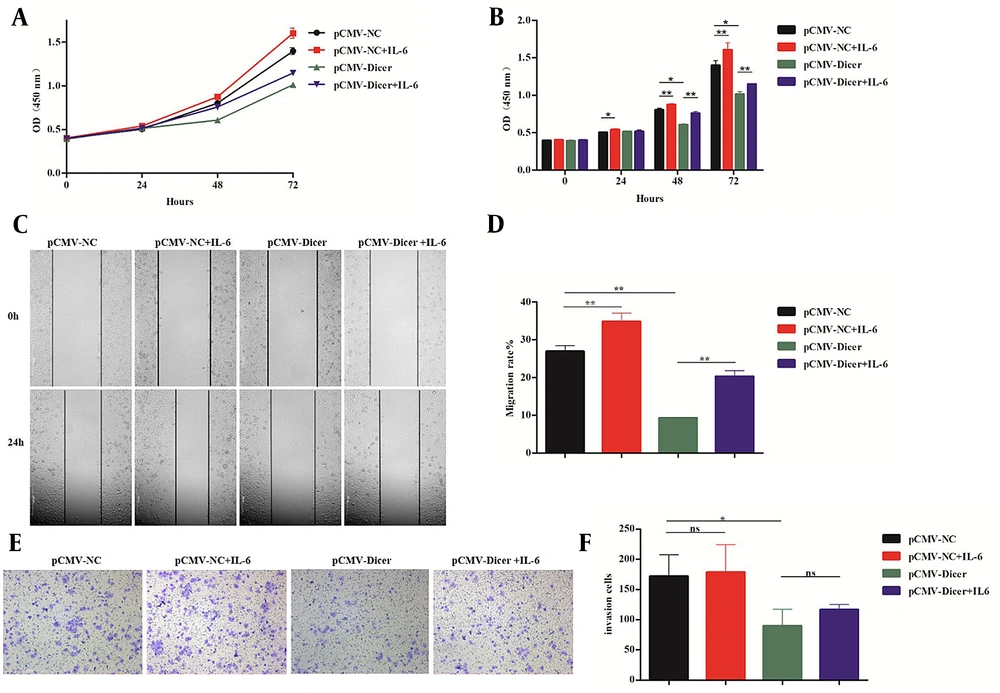
Interleukin 6 reversed the inhibitory effect of Dicer on hepatocellular carcinoma. A, the proliferation curve measured by the cell counting kit-8 (CCK-8) assay for the pCMV-NC cell incubated with interleukin 6 (IL-6) and pCMV-Dicer cell incubated with IL-6; B, the error bar chart of the CCK-8 assay of A; C, the migration evaluation by the wound scratch assay for the pCMV-NC cell incubated with IL-6 and pCMV-Dicer cell incubated with IL-6; D, the error bar of the migration rate of C; E, the invasion ability detected by the transwell test for the pCMV-NC cell incubated with IL-6 and pCMV-Dicer cell incubated with IL-6; F, error bars represent invasive cells. * P < 0.05, ** P < 0.01.
After co-cultured with RhIL-6 from 24 to 72 hours using the concentration of 20 ng/mL, the cell proliferation ability of both pCMV-NC from 24 to 72 hours (Figure 2A and B; P < 0.05) and pCMV-Dicer groups from 48 to 72 hours (Figure 2A and B; P < 0.01) increased compared to those without IL-6. The cell migration of both pCMV-NC (Figure 2C and D; P = 0.008) and pCMV-Dicer groups (Figure 2C and D; P = 0.006) also increased compared to those without IL-6, while the invasion remained unaffected for pCMV-NC (Figure 2E and F; P = 0.85) and pCMV-Dicer (Figure 2E and F; P = 0.18) with or without IL-6 addition. These data demonstrated that IL-6 reversed Dicer-induced HCC inhibition related to proliferation and migration in vitro.
The IL-6 receptor mono-antibody of tocilizumab with a concentration of 100 μg/mL was used to evaluate its effect on Dicer-related HCC inhibition. The proliferation of both pCMV-NC and pCMV-Dicer groups decreased from 24 to 72 hours (Figure 3A and B; P < 0.05) upon tocilizumab incubation. The migration of pCMV-NC (Figure 3C and D; P = 0.001) and pCMV-Dicer (Figure 3C and D; P = 0.007) was inhibited by tocilizumab. Also, the invasion of pCMV-NC (Figure 3E and F; P = 0.031) and pCMV-Dicer (Figure 3E and F, P = 0.001) significantly decreased upon tocilizumab incubation. Additionally, the decline of the pCMV-Dicer group upon tocilizumab incubation was more obvious than that of the pCMV-NC group related to proliferation (Figure 3A and B; P < 0.01), migration (Figure 3C and D; P = 0.000), and invasion (Figure 3E and F; P = 0.006). Unlike IL-6, tocilizumab enhanced Dicer-induced HCC inhibition, indicating that Dicer inhibits HCC by the IL-6 pathway.
Tocilizumab enhanced the inhibition of Dicer on hepatocellular carcinoma. A, the proliferation curve measured by the cell counting kit-8 (CCK-8) assay for pCMV-NC and pCMV-Dicer cells incubated with tocilizumab; B, the error bar of the CCK-8 assay of A; C, the migration evaluation by the wound scratch assay for pCMV-NC and pCMV-Dicer cells incubated with tocilizumab; D, the error bar represents the migration rate; E, the invasion ability detected by the transwell test for pCMV-NC and pCMV-Dicer cells incubated with tocilizumab; F, error bars represent the invasive cell quantity of E. * P < 0.05, ** P < 0.01.
4.2. Apatinib Cooperated with Dicer for HCC Suppression by Inhibiting IL-6
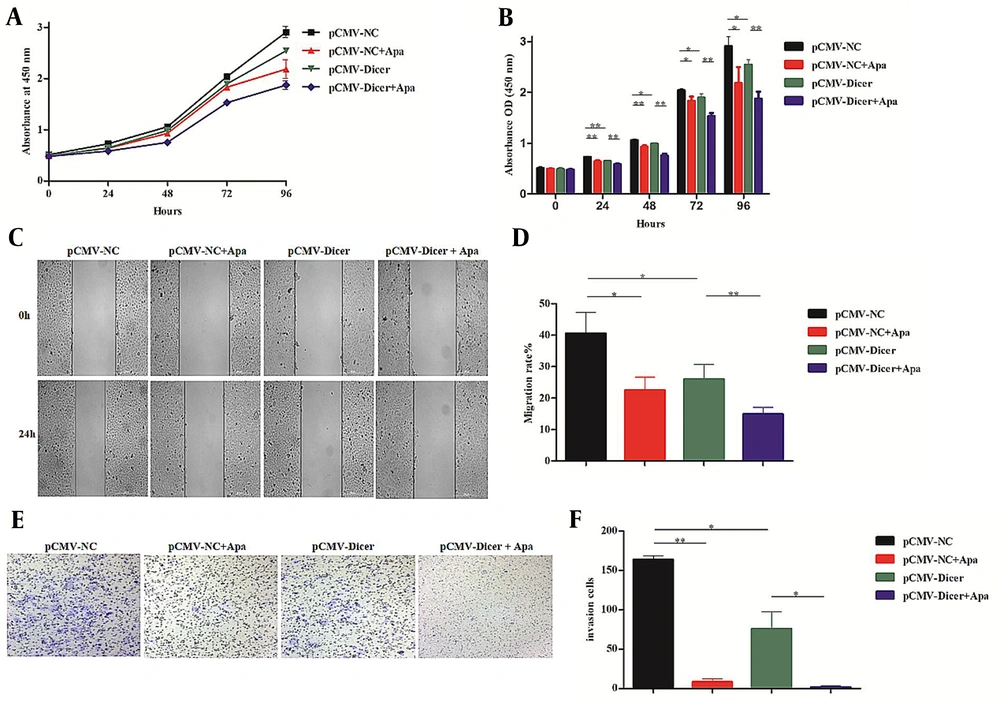
Since we previously confirmed that Dicer downregulated VEGF-A levels in HCC cells (26, 27), a VEGFR-2 inhibitor of apatinib used for HCC treatment was checked for its cooperation with Dicer. The results showed that apatinib with a concentration of 20 μM could suppress the proliferation from 24 to 96 hours (Figure 4A and B; P < 0.05), migration (Figure 4C and D; P = 0.016), and invasion ability (Figure 4E and F; P = 0.001) of pCMV-NC. However, pCMV-Dicer combined with apatinib displayed a more pronounced decline in proliferation from 24 to 96 hours (Figure 4A and B; P < 0.01), migration (Figure 4C and D; P = 0.002), and invasion (Figure 4E and F; P = 0.004) compared to pCMV-Dicer. These data indicate that apatinib cooperated with Dicer for HCC inhibition.
Dicer enhanced the inhibition of apatinib on hepatocellular carcinoma. A, the proliferation curve detected by the cell counting kit-8 (CCK-8) assay in pCMV-NC and pCMV-Dicer cells incubated with apatinib; B, the error bar of the CCK-8 assay of A; C, the migration assay by the wound healing test for pCMV-NC and pCMV-Dicer cells incubated with apatinib; D, error bars represent the migration rate of C; E, the invasion ability tested by the transwell assay for pCMV-NC and pCMV-Dicer cells incubated with apatinib; F, error bars represent the invasive cell number of E. * P < 0.05, ** P < 0.01.
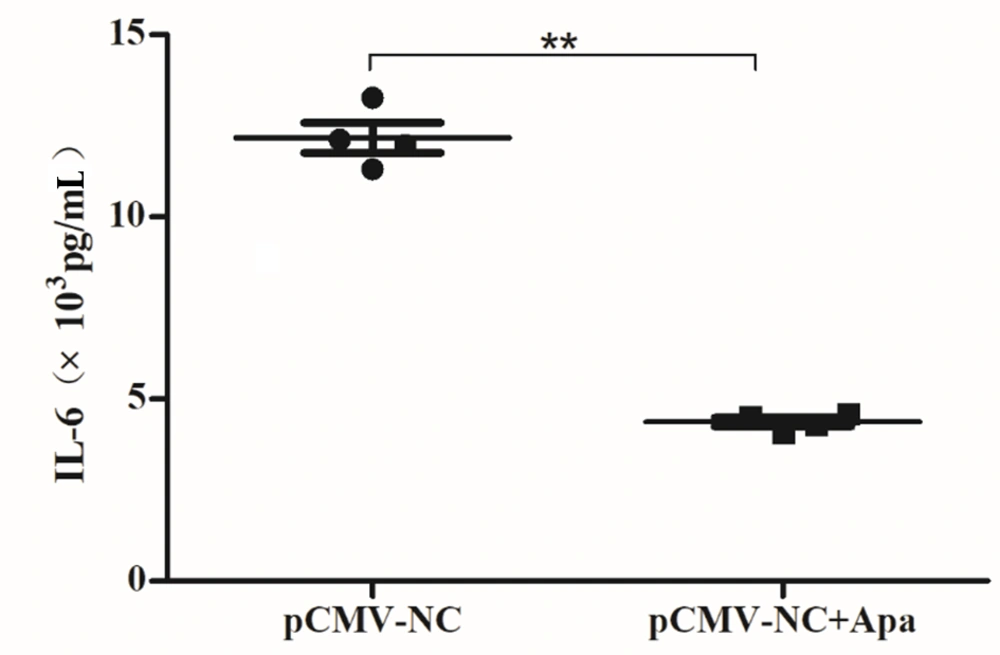
To investigate whether their cooperation was mediated by the IL-6 pathway, the medium of HCC cells incubated with apatinib for 72 hours was used for IL-6 measurement. As shown in Figure 5, IL-6 levels significantly decreased upon apatinib incubation (Figure 5; P = 0.000). These data implied that apatinib cooperated with Dicer for HCC suppression by downregulating IL-6.
Apatinib suppressed the level of interleukin 6 in hepatocellular carcinoma. The conditioned media of pCMV-NC and pCMV-Dicer cells incubated with apatinib for 24 hours were collected to assess the level of interleukin 6 (IL-6) by the flow cytometry/immunomagnetic beads (P = 0.000). ** P < 0.01.
5. Discussion
Many studies have reported the increased serum IL-6 in patients with various liver diseases, such as hepatitis B virus (HBV)- and hepatitis C virus (HCV)-related hepatitis (43), alcoholic cirrhosis (44), and HCC (45). The plasma levels of IL-6 increased with the progress of chronic liver disease and significantly correlated with the disease severity (44). Hepatitis B virus X protein (HBx) stimulates parenchymal liver cells to produce IL-6 in a MyD88-dependent manner that participated in HBV-mediated liver carcinogenesis (46). This cytokine is produced more in HCC patients than in cirrhosis patients and healthy controls (47). These reports are consistent with our finding that IL-6 weakened Dicer-induced HCC inhibition and caused more aggressive HCC cells.
The increase of Dicer could reduce the production of IL-6 and tumor necrosis factor α (TNF-α) in macrophages by upregulating miR-34a-5p (48), which is generally considered an effective tumor suppressor participated in p53-mediated tumor inhibition as a direct target of p53 (49). However, the reduced Dicer could increase IL-6 expression by downregulating miR-148a-3p, miR-152-3p, and miR-132 (50). The miRNA processing enzymes of Dicer might downregulate IL-6 expression by changing the related microRNA expression.
Tocilizumab suppressed oral squamous cell carcinoma (OSCC) and head and neck cancer in mice (51, 52). We also found that it could significantly enhance the Dicer-mediated decrease of growth on HCC. All of these suggest that tocilizumab might be a synergist for clinical HCC treatment. We evaluated the Dicer-IL-6 pathway on epithelial-mesenchymal transition (EMT), but no consistent results could be found upon Dicer and/or IL-6 incubation (data not shown).
Interleukin 6 involved in sorafenib and regorafenib drug resistance in HCC (53). The fact that apatinib inhibits IL-6 expression of HCC cell provided a feasibility that applying apatinib for sorafenib-resistant or regorafenib-resistant HCC patients. The synergistic effect of apatinib and Dicer on IL-6 downregulation implied that Dicer inducers would be a potential enhancer for apatinib related to HCC treatment.
5.1. Conclusions
Dicer could inhibit HCC by the IL-6 pathway, which would be a potential target for HCC treatment. The Dicer inducer might be an enhancer of apatinib for HCC treatment.