1. Background
According to a recent modeling study, when the global prevalence of viremic HCV infection is accepted as 0.7%, 56.8 million are thought to be infected with HCV (1).
The estimated prevalence of HCV is 0.2 - 0.5% in the United States and Western Europe and 1 - 3% in Japan (2, 3). The prevalence of HCV in the normal population in Turkey is 1% (4). In some European countries, countries such as Japan and Taiwan, the prevalence of HCV is high in the elderly population (5).
Pegylated interferon (PEG IFN) and ribavirin (RBV) combination therapy has been associated with a higher treatment discontinuation rate due to longer treatment duration and side effects (5). Among the various approved DAA regimens, first-line treatments are recommended for those with pangenotypic activity. DAA regimens simplify treatment as they eliminate the need for HCV genotyping and have high genetic antiviral efficacy (6, 7). Unfortunately, access to pangenotypic DAA in many countries is still limited by high treatment costs (8).
Glecaprevir/pibrentasvir (G/P) was approved in July 2017 for treating chronic hepatitis C in adults. G/P consists of a fixed-dose combination of two pangenotypic direct-acting antivirals (DAA) independent of RBV. Glecaprevir is an HCV NS3/4A inhibitor, and pibrentasvir is an NS5A inhibitor. Take three (100/40 mg tablets) once a day with food. The treatment duration is 8,12,16 weeks according to previous HCV treatment experience, cirrhosis, and HCV genotype status (9). G/P was approved for the first time in Turkey by the Ministry of Health of the Republic of Turkey for use in genotypes 2 and 3 in March 2019 and September 2019 (10). High SVR-12 rates have been reported between 95% and 100% in many clinical studies (11-17).
2. Objectives
Patients receiving treatment in real life may differ from those enrolled in clinical trials. This article investigates the efficacy, safety, and real-life results of glecaprevir/pibrentasvir, a new DAA that was applied to the Infectious Diseases Clinic and was prescribed chronic HCV treatment in the Turkish population.
3. Methods
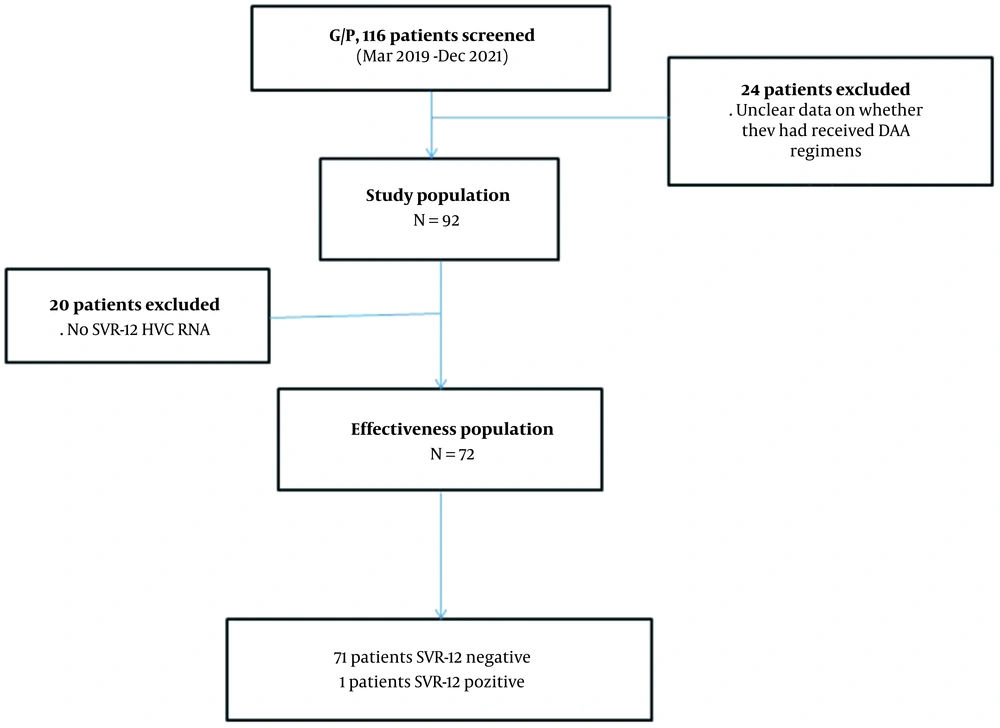
In this retrospective, observational, single-center study, 116 patients treated with glecaprevir/pibrentasvir for HCV between March 2019 and December 2021 by the Infectious Diseases and Clinical Microbiology clinic were included. Hatay is a province of Turkey in the Mediterranean Region with coasts at the eastern end of the Mediterranean Sea. This referral center is the only center providing HCV treatment in the Hatay region. The patients included in the study are shown in the flow chart (Figure 1).
For viral load determination, HCV–RNA levels were studied via real-time PCR (COBAS AmpliPrep/COBAS Tagman, Roche Diagnostics, Germany), and HCV genotypes were studied using a real-time HCV Genotype II (Anatolia geneworks, Turkey) system. The lower detection limit of the HCV RNA assay kit was 50 copies/mL.
Sustained virological response (SVR-12) was described as the inability to detect HCV viral load 12 weeks after treatment completion. Efficiency assessments other than SVR-12 were defined as follows. Early virological response (EVR): Absence of serum HCV RNA after 4 weeks of treatment. Virological breakthrough: Detection of previously undetected HCV RNA during treatment. Relapse: Detection of HCV RNA was not detected at the end of treatment or during follow-up after treatment. EVR and SVR-12 treatment efficacy analyses were performed between groups intended to treat (ITT) and per protocol (PP). Patients who completed the treatment period and who had HCV RNA test results in the 12th week after the end of treatment were included in the PP analysis. In addition to the pretreatment of HCV RNA levels, patients with at least one HCV RNA test result were included in ITT analysis. All cases with unknown sustained viral responses (SVR-12) were considered unresponsive in ITT analysis.
3.1. Statistical Analysis
IBM SPSS version 21.0 statistical package program (SPSS Inc, Chicago, IL, USA) was used for statistical analysis. The suitability of variables to a normal distribution was tested using the Shapiro–Wilk test and histogram. Mean and standard deviation were used for variables normally distributed, and median (median) and interquartile range were used for variables not normally distributed. This study was performed with the approval of the Mustafa Kemal University Faculty of Medicine Retrospective Ethics Board (reference number: 16.06.2022-19).
4. Results
During the study period, one hundred sixteen patients were prescribed glecaprevir/pibrentasvir (G/P). However, 20.7% (n = 24) of the patients were not included in the study because they did not come to follow-up and treatment despite a drug report and prescription.
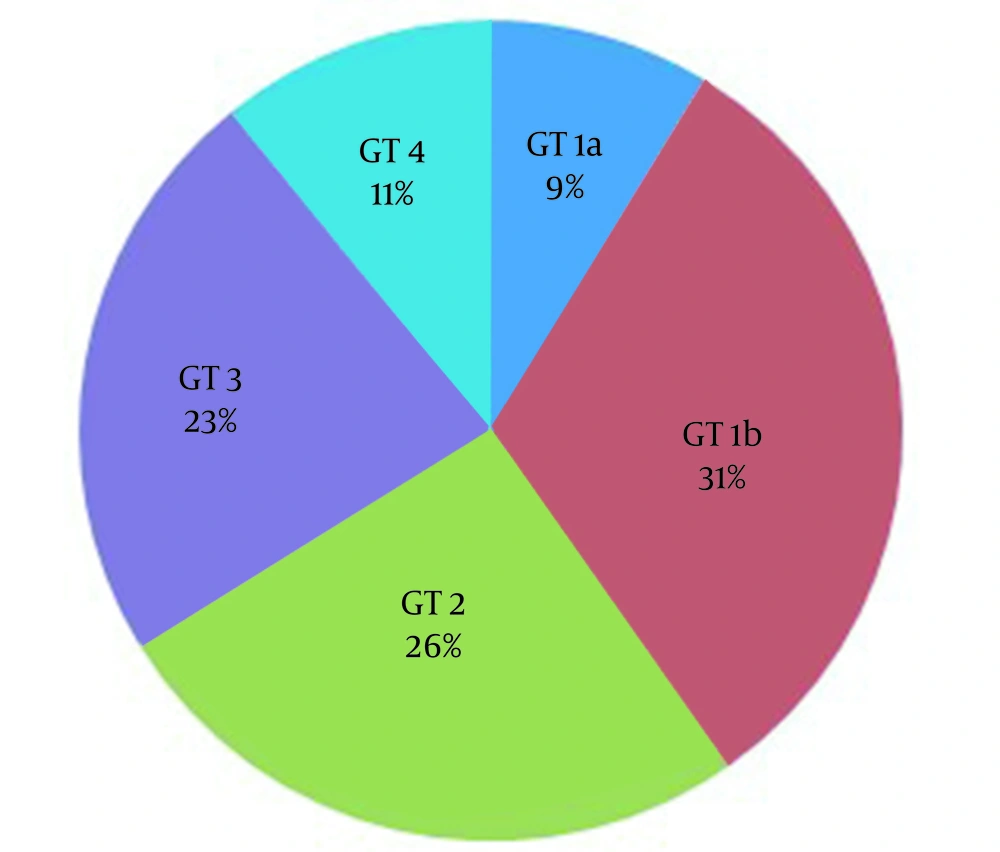
Ninety-two cases meeting the inclusion criteria were included in the study. 77.2% (71) of the patients were male, and 22.8% (n = 21) were female, with a mean age of 47.4 (18 - 89). Table 1 shows the patients' demographic, primary clinical characteristics, and biochemical and hematological parameters. 8.7% (n = 8) of the patients had previous treatment experience. Treatment-experienced patients; three peg interferon + ribavirin, two sofosbuvir + ribavirin, two dasabuvir + ombitasvir/paritaprevir/ritonavir and one of them glecaprevir + pibrentasvir treatment. The most common genotype (31.5%; n = 29) was 1b. Figure 2 shows the genotype distribution of cases. There were no cirrhotic patients in our study. Of the patients included in the study, 30.4% (n = 28) had a prison history, 40.2% (n = 37) had a history of substance use, and almost all of these patients had a history of IV drug use.
| Variables | Values (n = 92) |
|---|---|
| Age | 47.4 (18 - 89) |
| Gender, male | 71 (77.2) |
| Cirrhosis | 0 |
| Treatment-experienced | 8 (8.7) |
| Genotype 1b | 29 (31.5) |
| HCV RNA level | 222786 (2021 - 5378000000) |
| Biochemical parameters | |
| ALT | 92 (12 - 673) |
| AST | 54 (10 - 403) |
| BIL | 0.84 (0.3 - 0.5) |
| ALB | 4.4 (0.6 - 8.07) |
| CREA | 0.99 (0.3 - 9.09) |
| Hematological parameters | |
| HGB | 14.5 (8.8 - 18.2) |
| PLT, 103/mm3 | 233 (75 - 764) |
| INR | 1.05 (0.92 - 1.48) |
Abbreviations: HCV RNA, hepatitis C virus ribonucleic acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BIL, bilirubin; ALB, albumin; CRE, creatinine; HGB, hemoglobin; PLT, platelet; INR, international normalized ratio.
a Data expressed as No. (%), median (IQR) or mean ± SD.
Early virological response (EVC) was evaluated in 81 cases, and the EVC was 79/81 (97.5%). A total of 11 cases did not come to follow-up examinations for EVR. EVR could not be obtained from two patients. It was determined that these two patients were male and substance addicted. One was infected with genotype 3, and the other was infected with genotype 2. SVR-12 was obtained in these two patients.
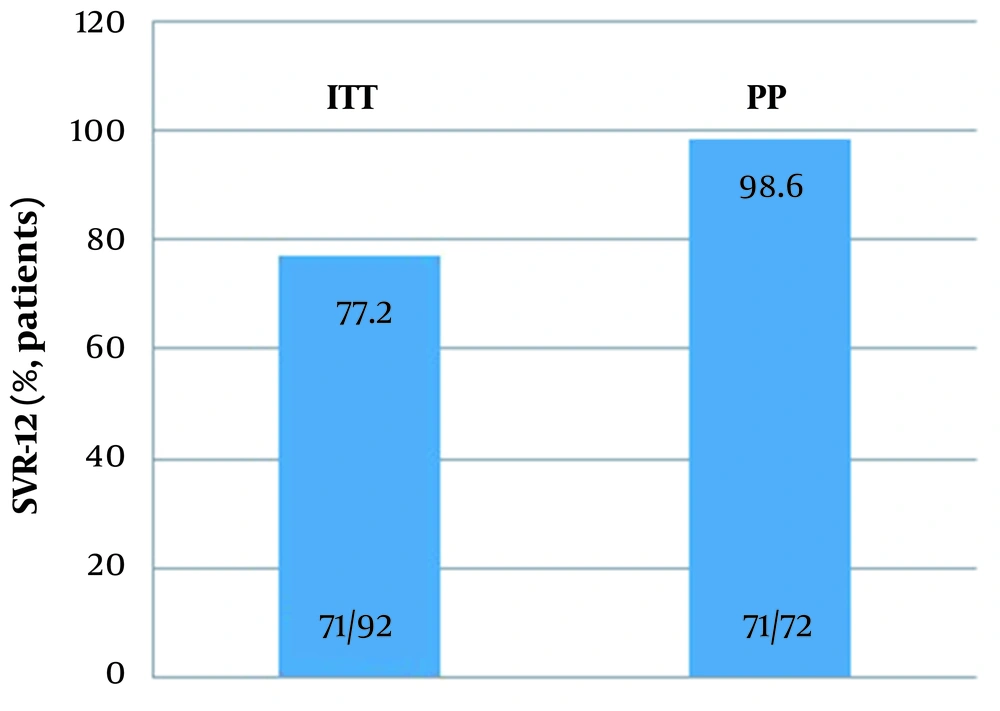
SVR-12 could not be obtained in only one of the patients. 92 and 72 cases were included in the ITT and PP populations, respectively, for treatment outcome analysis. PP analysis resulted in 98.6% (71/72), and ITT analysis resulted in 77.2% (71/92). Figure 3, SVR 12 rates according to G/P regime. In our study, SVR-12 could not be studied in 20 patients because although these patients achieved a virological response at the end of the treatment, they did not come to their follow-ups in the 12th week after treatment.
A 43-year-old male patient who could not obtain SVR-12. This patient had no previous treatment experience and was infected with genotype 1a. This patient was also receiving tenofovir disoproxil therapy for chronic hepatitis B virus infection. In addition, when the patient records were examined, the patient had a history of substance use, tattooing, and convictions. This patient was followed by the psychiatry clinic with the diagnosis of generalized anxiety disorder. No patient with relapse or virological breakthrough was detected in our follow-ups.
Of the 92 cases, 21 had hypertension, 11 had coronary artery disease, 10 had diabetes mellitus, 10 had chronic obstructive lung disease, 7 had benign prostatic hyperplasia, 5 had cerebrovascular diseases, 4 had osteoporosis, 2 had chronic hepatitis B infection, and 2 had chronic renal failure. In this study, no side effects were observed that would interrupt the treatment in any of the patients. The most common side effects are fatigue, itching, insomnia, and abdominal pain.
5. Discussion
This study is the first real-life data in our region, and although it was during the Covid-19 pandemic, high SVR-12 was obtained in patients with HCV. Three patients previously treated with NS5A received treatment for 16 weeks, and 89 patients for 8 weeks. No patients were treated for 12 weeks. During the study period, 116 patients' G/P was prescribed. However, 20.7% (n = 24) of these patients did not come for follow-up and treatment despite a drug report being issued for treatment. The reason for this may be due to the difficulties patients experience in reaching the health system due to being in the Covid-19 pandemic period. Another reason may be that 18 cases were convicted when the patient records were examined and that these patients had difficulty reaching the hospital from prison during the pandemic. In addition, three of the other six patients may not have been included in the healthcare service because of their substance abuse and the other three's advanced age (89,90,89 years).
In our study, high-efficiency SVR-12 rates (98.6%) were obtained in all genotypes, and SVR-12 could not be obtained in only one patient. This patient was a genotype 1a infected, treatment-inexperienced, and noncirrhotic patient. In a real-life study conducted in the Italian population, SVR-12 was achieved at 99.3%, with treatment failure in five patients and relapse in one patient. In addition, it has been shown that being infected with the male gender and genotype 3 is associated with low SVR-12 (18). In a real-life study conducted in the German population, SVR-12 was 96.7%, virological failure was detected in one patient, reinfection was detected in two patients, and there was no patient with relapse (19). In a real-life study conducted in the Taiwanese population, 8-16 weeks of G/P were effective and tolerated in patients with chronic HCV infection (20).
In a real-life study conducted by Çölkesen et al. in Turkey with 127 patients receiving G/P treatment, they found that 92.9% (n = 118) of the patients were injecting (IV) drug use and 61.4% (n = 78) of the patients were convicted. The most frequently detected genotype was 83.6% (n = 106) genotype 3. The virological response was achieved in 99.2% (n = 126) of patients, and only one patient did not achieve the end-of-treatment response and SVR-12 (21). In another study examining the rates of IV use and the results of direct-acting antiviral treatment in convicted patients in Turkey, the most common viral genotype 3 (41.6%) and genotype 4 (39.0%) were found (22). In our study, 23% (n = 21) of patients infected with genotype 3 were treated, SVR-12 was obtained in all patients, and no patients with relapse or reinfection were detected. As a result, genotype 3 is more common in convicted patients in Turkey, mainly due to IV drug use and substance use. In addition, in the study conducted by Çölkesen et al., the mean age of the patients was 27, and it was 47 in our study. This age difference may be because most of the patients are young patients convicted and addicted to IV drugs.
In a study conducted in Austria, excellent agreement was achieved with G/P given as a directly observed treatment in IV drug users, and it resulted in high rates of SVR-12 (94.6%) similar to other patients (23). However, Gonzalez-Serna et al.'s study evaluated the SVR-12 of G/P between individuals using and not using IV active drugs. Active IV drug use has been associated with lower rates of SVR due to higher voluntary drug withdrawal (24). In our study, 30% (n = 28) of the patients were convicted, 40% (n = 37) were patients using intravenous drugs, and high SVR-12 was obtained in these patients. It is understood from these studies that SVR-12 rates were similar to the normal population when people using intravenous drugs were provided with regular use of their treatment.
In a study conducted in Asia, HCV genotype 2 was found most frequently (25). In a study conducted in Turkey, the most common HCV genotype 1 was detected (4). Especially in Turkey, genotypes 3 and 4 have started to be seen frequently in the convicted population with intravenous drug use (21, 22). The fact that the patients have been treated recently and that they are convicted and using IV drugs at the same time may explain the changes in this genotype distribution. The fact that a significant portion of the patients in our study was convicted and using IV drugs may be the main reason for the genotype change. As a result, genotype changes stand out, depending on the geographical region and the changes in risk groups.
In a study conducted in Asia, itching, anorexia, and fatigue were found to be the most common side effects (SE). Nine serious SEs unrelated to G/P occurred. They detected a grade 3 elevation in the bilirubin level in three patients. Early treatment discontinuation, hepatic decompensation, or death were not observed in these patients (25). In a real-life study in Taiwan, the two most common SEs were itching and fatigue. AST and total bilirubin levels were not increased three times above the upper limit of normal in any of the patients (20). In a real-life study conducted in the German population, at least one treatment-related SE was reported in 8% (n = 60); fatigue, itching, nausea, and headache were the most common. Although four patients stopped treatment early due to SEs, SVR-12 was obtained (19). In our study, no patient discontinued treatment early due to SE. The most common SE were fatigue, itching, insomnia, and abdominal pain. Consequently, more real-life data are needed to evaluate the effectiveness and reliability of G/P.
5.1. Study Limitations
The limitation of our study is that it is a retrospective study, the data is regional, and the number of cases is low.
5.2. Conclusions
This study made us think that G/P therapy is used with very high efficiency and tolerability in real life in our country, despite being in the COVID-19 pandemic period. In addition, a significant change was observed in the genotype distribution previously reported from our country in the patient group we treated.