1. Background
Hepatitis C is one of the liver infections spread by contact with blood and through needles or other equipment from infected persons (1, 2). Hepatitis C usually is a short-term illness; however, in some cases, it can become a long-term chronic infection and, consequently, serious diseases, such as cancer and liver cirrhosis (3-5).
According to a World Health Organization report, it has been estimated that 58 million individuals suffer from chronic hepatitis C virus (HCV) infection. About 1.5 million new cases occur annually, and 3.2 million individuals (adolescents and children) live with chronic hepatitis C infection. Additionally, it has been estimated that about 290,000 individuals died from hepatitis C in 2019, most of which were due to cirrhosis and hepatocellular carcinoma (HCC) (6). According to studies, the overall seroprevalence of HCV in the general population in Iran was 0.6%, which ranged from 0.08% to 1.6% based on different provinces (7). In a study in 2014, the number of individuals suffering from HCV infection was estimated to be around 186,500 in Iran (8). The modeling results showed that assuming the current settings of diagnosis/treatment, it is expected that by 2030, the number of HCV cases will increase to 213,700, and the number of decompensated cirrhosis, HCC, and liver disease mortalities will increase threefold to fourfold in Iran (8).
Cirrhosis is characterized histologically by the formation of diffuse nodules surrounded by dense fibrotic lamina, along with hepatic tissue disruption, collapse, and rupture of the vessels around these laminae (9, 10). In addition to hepatitis C, the leading causes of cirrhosis in more developed countries are hepatitis B virus (HBV) infection, alcohol abuse, and increased non-alcoholic fatty liver disease (NAFLD) (11). Although the standard method for diagnosing and examining liver cirrhosis is a biopsy, this process is invasive, costly, and unpleasant for the patient. It can be accompanied by serious complications, such as death (12, 13). Therefore, replacing a non-invasive and effective method in predicting cirrhosis seems necessary.
The global prevalence of liver cirrhosis in biopsy studies ranges from 4.5% to 9.5% in the world’s population (14). According to the Global Burden of Diseases study (2016), cirrhosis was responsible for approximately 37 million years of life lost in that year, an increase of 7.1% from 2006 to 2016 (15, 16).
Liver cirrhosis is considered an important stage during the history of hepatitis C infection. It is important to pay special attention since it is associated with high mortality and imposes a high economic burden on the health system. Liver cirrhosis prevention, diagnosis, and treatment are essential. Diagnosing factors predicting cirrhosis in these patients is important in preventing serious diseases, such as HCC and gastroesophageal varices, and the relative reduction of liver failure and liver transplant cases (17).
2. Objectives
As no study has been conducted to determine the predictive factors of liver cirrhosis in patients with hepatitis C infection in Iran, this study aimed to determine the predictors for liver cirrhosis in patients with hepatitis C infection.
3. Methods
3.1. Patients and Study Designs
The present cross-sectional study was performed on all patients referred to a tertiary referral center (Rasool Akram), Iran University of Medical Sciences, Tehran, Iran, from the beginning of 2011 to the end of 2017 with a definite diagnosis of HCV. After applying the inclusion and exclusion criteria, these patients were included in two groups with cirrhosis and without cirrhosis. The Ethics Committee of Iran University of Medical Sciences approved the design and all stages of the study (IR.IUMS.FMD.REC.1399.489). This study’s research team adhered to the Helsinki Convention ethical principles regarding clinical studies in all stages of the present study. This study involved a retrospective review of medical records, and the requirement for informed consent was waived.
3.2. Eligibility Criteria
The inclusion criteria were HCV-positive confirmation based on enzyme-linked immunosorbent assay and HCV ribonucleic acid (RNA) polymerase chain reaction, interpretable imaging (positive fibro scan result), histological findings (liver biopsy), elevated serum alanine transaminase (ALT) levels, the existence of thrombocytopenia, and filling of a consent form. The exclusion criteria were the presence of human immunodeficiency virus (HIV) antigen, no information of the duration of HCV infection based on the patient’s statements and autoimmune, tumor, and biliary or vascular liver diseases.
3.3. Patient Grouping
The patients were classified into cirrhosis (the case group) and non-cirrhosis (the control group) based on the presence of cirrhosis. The diagnosis of cirrhosis was confirmed based on imaging findings (positive fibro scan result), histological findings of the liver (liver biopsy), thrombocytopenia presence, and direct observation of esophageal varices in the upper endoscopy.
3.4. Variables and Data Collection
In both groups, patients’ demographic data, including age, gender, place of residence, ethnicity, diabetes, alcohol consumption, smoking, drug addiction, immunosuppressive status (including HIV and organ transplantation), and family history of liver disease, including hepatitis B, cirrhosis, and HCC, were collected by referring to patients’ files using a checklist. Laboratory data were collected by referring to patients’ records, including liver function tests, blood group, aspartate transaminase (AST), ALT, prothrombin time (PT), partial thromboplastin time (PTT), fasting blood sugar, anti-HCV antibodies, platelet count, and virus count. Finally, laboratory findings and demographic characteristics were compared between the two groups.
3.4.1. Sample Size Calculation
The appropriate sample size to conduct this study, with an estimated effect size of 0.42, for the correlation of predictive factors of liver cirrhosis in patients with hepatitis C based on a study by Vaz et al. (18), with an alpha error of 5% and a power of 80%, by an epidemiologist, using G*Power software (version 3.1), was estimated 85participants for each group and a total of 116 participants.
3.5. Statistical Analysis
The data were entered into SPSS software (version 22). The mean and standard deviation or amplitude of quartile deviation was used to describe the measured quantitative variables. The chi-square test was used to examine qualitative variables. A t-test was used to evaluate the relationship between quantitative variables in the two groups if the data distribution was normal. If the distribution is not normal, the non-parametric Mann-Whitney U test was used. Numerous univariate and multivariate logistic regression analyses were performed to achieve a regression coefficient and odds ratio and determine the most important predictors of cirrhosis. A P-value < 0.05 was considered statistically significant.
4. Results
4.1. Demographic Characteristics
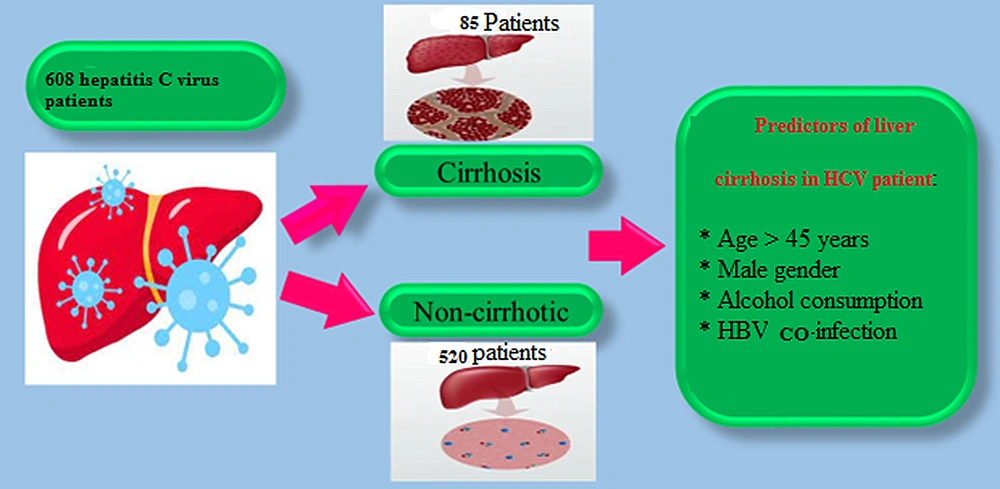
A total of 608 patients with a definitive diagnosis of hepatitis C were included in the study, 424 (69.7%) and 184 (30.3%) of whom were male and female, respectively. The prevalence of liver cirrhosis was 13.9% (n = 85). The patients’ mean age was 43 ± 13 years (range: 21 - 82 years). Additionally, 535 patients (88%) were married, and 262 subjects (43%) were unemployed. In this study, 227 patients (37.3%) had a type O blood group. The mean age values were 46 ± 19 and 42 ± 17 years in the cirrhosis and non-cirrhosis groups, respectively, with no statistically significant difference (P > 0.05). Moreover, 80% of patients with cirrhosis were male. Compared to the non-cirrhosis group, the proportion of male patients was significantly higher in the cirrhosis group (P = 0.026).
The co-infection with hepatitis B was associated with liver cirrhosis (P < 0.05). There was no significant difference in smoking history, occupational status of patients, type of blood groups, injecting drug addiction, and marital status between the two groups (P > 0.05) (Table 1).
| Factors | Cirrhosis (n = 85) | Non-cirrhosis (n = 523) | P-Value |
|---|---|---|---|
| Age (y) | 46 ± 19 | 42 ± 17 | 0.24 |
| Age (y) | 0.021 | ||
| < 45 | 27 (26.5) | 173 (33.1) | |
| ≥ 45 | 66 (74.5) | 350 (66.9) | |
| Gender | 0.026 | ||
| Male | 68 (80) | 345 (66.1) | |
| Female | 17 (20) | 178 (33.9) | |
| Occupation | 0.41 | ||
| Employed | 29 (34.1) | 188 (36.1) | |
| Unemployed | 23 (65.9) | 335 (63.9) | |
| Blood group | 0.21 | ||
| O | 30 (35.2) | 197 (37.6) | |
| A | 25 (29.4) | 141 (27.1) | |
| B | 19 (22.3) | 109 (21.1) | |
| AB | 11 (13.1) | 76 (14.2) | |
| Family history (positive) | 6 (7) | 32 (6.1) | 0.66 |
| Smoking (positive) | 11 (12.9) | 58 (11.08) | 0.53 |
| Marital status | 0.88 | ||
| Married | 72 (84.7) | 463 (88.5) | |
| Unmarried | 13 (15.3) | 60 (11.5) | |
| Injection addiction (positive) | 8 (9.4) | 19 (3.6) | 0.085 |
| Alcohol consumption (positive) | 31 (36.4) | 88 (16.8) | 0.001 |
4.2. Univariate Analysis of Variables
The univariate analysis of laboratory findings showed that the level of hepatic enzyme AST in the cirrhosis group (60.28 ± 23.3) was significantly higher than in the non-cirrhosis group (51 ± 22.8) (P = 0.032). The mean level of the liver enzyme ALT in cirrhosis patients was significantly higher than in non-cirrhosis patients (78.36 ± 35.8 and 57 ± 33.1) (P = 0.021). Platelet levels were significantly lower in cirrhosis patients (P = 0.028). Insulin-resistant diabetes showed a significant relationship with cirrhosis. Accordingly, the number of patients with insulin-resistant diabetes was higher in the cirrhosis group (P = 0.01). The levels of PT, PTT, and international normalized ratio (INR) in cirrhosis patients were significantly higher than in non-cirrhosis patients (P < 0.05). The mean HCV RNA level in cirrhosis patients was significantly higher than in non-cirrhosis patients (P = 0.001). There was no significant difference in cholesterol and hemoglobin levels between patients in the two groups (P > 0.05) (Table 2).
| Factors | Normal Range | Cirrhosis (n = 85) | Non-cirrhosis (n = 523) | P-Value |
|---|---|---|---|---|
| Aspartate transaminase (IU/L) | 29 - 33 | 60.28 ± 23.3 | 51 ± 22.8 | 0.032 |
| Alanine transaminase (IU/L) | 10 - 40 | 78.36 ± 35.8 | 57 ± 33.1 | 0.021 |
| Platelet × 103 (g/dL) | 150 - 450 | 136 ± 183 | 216 ± 195 | 0.028 |
| Hemoglobin (g/dL) | 11 - 16 | 14.6 ± 1.6 | 15.1 ± 1.5 | 0.081 |
| Diabetes (positive) | 27 (31.7) | 88 (16.8) | 0.001 | |
| Prothrombin time (s) | 11 - 13.5 | 13.4 ± 4.8 | 11.8 ± 4.1 | 0.027 |
| Partial thromboplastin time (s) | 25 - 40 | 33.8 ± 15.9 | 27.1 ± 15.01 | 0.038 |
| International normalized ratio (s) | 0.8 - 1.1 | 1.9 ± 0.18 | 0.09 ± 1.02 | 0.001 |
| Cholesterol (mg/dL) | Less than 200 | 132.89 ± 83.2 | 121.2 ± 63.5 | 0.078 |
| Hepatitis B virus co-infection | 15 (17.6) | 47 (8.9) | 0.001 | |
| Hepatitis C virus ribonucleic acid level (log IU/mL) | 1.0 - 8.0 | 8.58 ± 2.7 | 5.01 ± 2.11 | 0.001 |
4.3. Multivariate Analysis
An adjusted logistic regression analysis was conducted to control for confounders for the assessment of the factors associated with cirrhosis in patients with hepatitis C. Multivariate analysis showed that age > 45 years, male gender, co-infection with HBV infection, and alcohol consumption were predictive factors for cirrhosis in patients with hepatitis C (Table 3). Figure 1 depicts a summary of the results.
| Factors | ORAdj | 95% CI | P-Valve |
|---|---|---|---|
| Age ≥ 45 y | 1.11 | 1.02 - 3.77 | 0.028 |
| Gender (male) | 2.08 | 1.21 - 3.11 | 0.023 |
| Alcohol consumption | 1.87 | 1.19 - 3.55 | 0.001 |
| Hepatitis B virus co-infection | 2.58 | 1.25 - 4.51 | 0.001 |
Predictors of Liver Cirrhosis in Hepatitis C Virus Patients Based on Multivariate Analysis a
5. Discussion
Cirrhosis is an important milestone in HCV history, as it indicates significant mortality and higher healthcare costs associated with end-stage liver disease complications. Due to the close association between HCV and cirrhosis, the present study was designed and performed for the first time in Iran to analyze the demographic, clinical, laboratory, and virological data associated with cirrhosis. Finally, the predictors of cirrhosis can be determined in patients with hepatitis C.
The demographic findings of the present study showed that the incidence of cirrhosis in individuals with HCV is significantly associated with four variables of age, gender, alcoholism, and co-infection with HBV. These findings are consistent with the results of studies conducted in this area. In 2020, Vaz et al. conducted a cohort study on cirrhosis incidence, etiology, and comorbidities in a Swedish population. The aforementioned study showed that cirrhosis incidence is 23.2 per 100,000 individuals annually, estimated at 30.5 and 16.4 in male and female cases, respectively. When the data were classified by age, the highest incidence was recorded at 60 - 69 years, and male cases had a higher incidence than female cases in most age groups. According to the results of the aforementioned study, the most common causes of cirrhosis were alcohol consumption (50.5%), cryptogenic cirrhosis (14.5%), hepatitis C (13.4%), and NAFLD (5.7%). Most patients had at least one liver-related complication at diagnosis (68%). Finally, Vaz et al. concluded that the increase in cirrhosis is multifactorial and is likely related to a higher incidence in older individuals (18).
In 2016, Nilsson et al. conducted a cohort study on southern Sweden’s cirrhosis prevalence, clinical manifestations, and mortality. The results of the aforementioned study showed that the most common causes of cirrhosis were excessive alcohol consumption (58%), HCV infection (13%), and cryptogenic cirrhosis (12%). When classified according to age and gender, the results of the aforementioned study showed that when these two factors are associated with HCV, the mortality rate due to cirrhosis increases significantly. The aforementioned study showed that old age and male gender significantly increase the mortality rate due to cirrhosis in patients with HCV (19).
The results of a study by Pol et al. in 2017 showed that the prevalence of cirrhosis in cases of concurrent HBV/HCV is significantly higher (11%) than in cases of one with HBV (2%) or HCV (4%). According to the results of the aforementioned study, a history of alcohol abuse was higher in patients with concomitant HBV/HCV (26%) than in patients with HBV alone (12%). Still, it was similar in patients with HCV alone (32%). Multivariate analysis in the aforementioned study confirmed the association between cirrhosis and co-infection of HBV and HCV (20).
The results of multivariate analysis in the present study showed that age over 45 years, male gender, co-infection with HBV infection, and alcohol consumption were the most important risk factors for cirrhosis in patients with hepatitis C. Very few studies have examined the predictors of liver cirrhosis in patients with hepatitis C. The current study showed that the level of laboratory factors was higher in cirrhosis patients than in non-cirrhosis patients. According to the comparison of the results of the current study to the results of similar studies, it is concluded that the present study’s results are consistent with the available evidence.
In a 2005 study by Lok et al., of 1141 enrolled patients, 429 subjects were cirrhosis patients. The aforementioned study showed that the three variables of platelet count, AST/ALT ratio, and INR were higher in cirrhosis patients than in non-cirrhosis patients in patients with HCV (21). High sample size (1114 versus 608) and multi-center and multi-racial subjects (10 centers in the United States with some races, such as black and white, versus one center with Iranian race) increase the aforementioned study’s power, compared to the present study. However, in the current study, the confirmation of other four variables (age over 45 years, male gender, alcohol consumption, and co-infection with HBV) in addition to the three variables in the aforementioned study can be considered a strength.
Sheth et al. showed that the mean AST/ALT ratio was higher in cirrhosis patients than in non-cirrhosis patients, a positive predictor of cirrhosis (22). In a systematic review by Freeman et al. in 2003 to predict the progression of cirrhosis in chronic HCV infection, they stated that male gender, high alcohol consumption, and histological evidence of progressive inflammatory activity are significantly related to cirrhosis and can be considered the predictors of cirrhosis (23).
Numerous studies have shown that HBV infection increases fibrosis in patients with chronic HCV (24, 25). One study that, more than any other, confirms the increased risk of cirrhosis in patients with concomitant HCV and HBV is a 1997 study by Roudot-Thoraval et al. The aforementioned study was performed in 143 medical centers in France, and 6664 patients were included. The aforementioned study showed that the route of virus transmission, alcohol abuse, and HBV infection are significantly associated with the risk of cirrhosis (26). This explanation could justify the findings of the present study confirming concurrent HBV infection as a predictor of cirrhosis in patients with HCV. In 2016, Mirminachi et al. conducted a methodologically similar study to the present study entitled “predictors of cirrhosis in chronic HBV infections” in Iran. In the aforementioned study, 237 patients were included. The results of the present study on ages above 45 years are similar to those of Mirminachi et al.’s study (7). It is noteworthy that gender and co-infection with HBV and HCV, which are confirmed as the predictors of cirrhosis in patients with HCV (in the current study), cannot be used as a predictor in patients with HBV (in Mirminachi et al.’s study (7)).
The present study had some weaknesses and strengths that should be pointed out. Due to the study’s retrospective nature and review of patients’ files, it was impossible to examine several important factors, such as the duration of hepatitis C and the history of receiving antiviral treatments, which can affect the study results. Additionally, for the laboratory factors, this study only reported the values recorded at the time of the visit to patients, which only indicated the progress of HCV infection, and it was impossible to measure the pathological and histological findings of the patients. A prospective study design with a large sample size can help estimate the results more accurately.
The strengths of this study include the 8-year study period and acceptable sample size. Comparing the existing studies in this field to the present study showed that the most serious limitation of this study might be the admission of patients in one medical center (compared to the studies that examined 10 and even 143 centers).
5.1. Conclusions
The present study’s results showed that the risk of liver cirrhosis can differ in HCV patients, depending on some demographic and clinical factors. In patients with hepatitis C, the factors of age > 45 years, male gender, alcohol consumption, and simultaneous HBV infection significantly increased the risk of liver cirrhosis. As these factors are strong predictors for cirrhosis in patients with hepatitis C, the diagnosis and treatment of these individuals can be an important step in preventing the morbidity and mortality of this disease. Cirrhosis in patients with HCV can be predicted with elementary, non-invasive, and cost-effective variables, which is clinically important in the better management of these patients. The results of the present study can be a guide for physicians to manage these patients better and give them priority in treatment.