1. Background
Hepatocellular carcinoma (HCC) is a common malignant tumor of the digestive system and is also the seventh most common cancer and the fourth leading cause of cancer-related death worldwide (1). There are no obvious early symptoms in the development of HCC, and extrahepatic metastases (EHM) already exist by the time most patients with mid-to-late-stage HCC are detected. Extrahepatic metastases occur mainly through blood and lymphatic vessels, with the most common site of EHM being the lungs, followed by bones and other areas (2). For the treatment of extrahepatic metastatic hepatocellular carcinoma (HCC-EHM), the National Comprehensive Cancer Network (NCCN) and Barcelona-Clinic Liver Cancer (BCLC) guidelines recommend systemic targeted immunotherapy, systemic chemotherapy, radiotherapy, and other symptomatic supportive treatments (3, 4).
Currently, sorafenib and targeted interventions are considered basic treatment options for advanced HCC, and recently immune checkpoint inhibitors have shown an important role in the targeted treatment of HCC (5). However, a study showed that surgery in the primary site provided a longer median survival than radiofrequency ablation (RFA) and transcatheter arterial chemoembolization (TACE) for patients with EHM (6). A recent study concluded that tyrosinase inhibitors (TKIs) and anti-PD-1 antibodies in patients with HCC-EHM enable reconsidering of surgical treatment modalities (7).
In a meta-analysis, Yang et al. showed that surgical treatment and microwave ablation were equally effective in patients with HCC-EHM (8). Some authors have suggested that most patients with HCC-EHM die from progressive intrahepatic tumors rather than EHM (9, 10). Although the current guidelines do not recommend surgical treatment, there is a group of patients who have EHM but have relatively small primary liver foci that are relatively easy to remove surgically, and this group of patients might achieve better results with surgical resection followed by a combination therapy. However, for patients with HCC-EHM, there are no clear criteria to assess whether patients can acquire an extended survival time after surgery.
2. Objectives
In this study, the SEER database was screened for HCC patients with EHM, and a nomogram was mapped for assessing and screening which patients are suitable for primary focal surgical treatment, predicting the probability that they will be able to have extended survival time and survival rates at 1, 3, and 5 years.
3. Methods
3.1. Materials
This study used the National Cancer Institute’s SEER 17-Registry (2004 - 2018 dataset), a database that includes information on the occurrence of various malignancies in most regions of the United States. Cases with histological subtypes of HCC were determined using the variable “ICD-0-3 Hist/Behav, malignant”. The type of local therapy for the primary tumor was identified using the codes of the variable “surgery of the primary site”. Types of surgical management include transplantation, segmental resection, lobectomy, and wedge resection. From 2004 - 2018, a total of 106,614 patients were identified. The exclusion criteria included (a) patients with lower than stage IV (i.e., no lymph node metastases and distant metastases; the American Joint Committee on Cancer (AJCC) 8th TNM staging); (b) a combination of other malignancies; (c) missing survival status; (d) unknown TNM staging; and (e) no pathological diagnosis. The basic information of the patient was extracted from the database, mainly including the year of diagnosis, age, gender, marital status, race, grade, T staging, NM staging, tumor size, alpha-fetoprotein (AFP), fibrosis score, radiotherapy, chemotherapy, and surgical treatment information of the primary site. The primary endpoints include overall survival (OS) and cancer-specific survival (CSS). We ultimately included data from 6239 patients in the analysis. This study was approved by the Ethics Review Committee of the First Affiliated Hospital of Chengdu Medical College (KY2023 - 088). Following the formal registration and application on the website, the approval and the credentials to have access to the data were warranted by the SEER organization.
3.2. Propensity Score Matching
Because patient inclusion was not randomized and imbalances in baseline characteristics could lead to selection bias, this study used patients in the surgical group matched to patients in the nonsurgical group. Several baseline covariates, including year of diagnosis, age, gender, marital status, race, grade, T staging, NM staging, tumor size, AFP, radiotherapy, chemotherapy, and fibrosis score, were considered in calculating propensity scores using a logistic regression model. The caliper value was set to 0.01 because this study used the SEER database, which has a large enough sample for the current analysis. A smaller caliper value reduces the sample size; however, again, it reduces the interference of other confounding factors to a greater extent, which can make the conclusions more accurate. Finally, 912 patients were successfully matched.
3.3. Statistical Analysis
Statistical analysis was performed using SPSS software (version 26.0) and R software (4.2.1). Continuous variables were transformed into categorical variables, and the chi-square test was used to compare clinicopathological and demographic data between the two groups. Survival curves were plotted using the Kaplan-Meier method, and differences were analyzed using the log-rank test. Multivariate survival analysis was performed using the Cox proportional hazard model. The Akaike information criterion (AIC) and the Bayesian information criterion (BIC) were calculated to select the best regression model. The receiver operating characteristic (ROC) curves were used to evaluate the predictive power of the nomogram. Then we plot the calibration curves of the training and validation groups. For patients in the surgical group, the predictions of 1-, 3-, and 5-year survival rates were also made. All statistical significance was defined as P < 0.05.
4. Results
4.1. Clinicopathological Characteristics of Patients
In the present study, a total of 6,238 patients with HCC were included. Of these cases, 513 patients (8.2%) were treated surgically; however, 5725 patients (91.8%) were not treated surgically. In the year of diagnosis, the number of patients in the non-surgical group increased between 2012 - 2019 (P < 0.001). More patients older than 60 years were non-surgically treated (P < 0.001). More male patients had HCC; nevertheless, only 386 male patients (7.6%) were treated surgically (P < 0.001). There was no statistical difference between the whites and other races in whether they received surgical treatment (P = 0.526). Only 20% of grade IV (13/65) patients underwent surgical treatment.
T staging showed a statistical difference between the surgical and non-surgical groups (P < 0.001). Only 5% (n = 154) of the patients with T3 staging underwent surgery, and 10.3% of patients with T4 stage underwent surgery. Regarding two aspects of EHM, patients with lymph node metastasis only (N1M0) had more chances to undergo surgery (n = 243, 13.7%) than those with distal organ metastasis (N0M1, n = 212, 6.7%). Notably, 58 patients (4.5%) with N1M1 staging underwent surgery. Most patients had tumors > 5 cm in size and were not treated surgically (P < 0.001). In patients treated surgically, a higher number were AFP negative or unknown (P < 0.001). Patients in the surgical group had lower hepatic fibrosis scores (0 - 4 points) (P < 0.001). Most patients in the surgical group did not receive radiation therapy (P < 0.001). Chemotherapy was not statistically different for this factor (P = 0.329) (Table 1).
| Variables | Surgical Group (n = 513) | Non-surgical Group (n = 5725) | P-Value |
|---|---|---|---|
| Year of diagnosis | < 0.001 | ||
| 2004 - 2011 | 290 (10.4) | 2505 (89.6) | |
| 2012 - 2019 | 223 (6.5) | 3220 (93.5) | |
| Age (y) | < 0.001 | ||
| ≤ 60 | 272 (10.1) | 2414 (89.9) | |
| > 60 | 241 (6.8) | 3311 (93.2) | |
| Gender | < 0.001 | ||
| Male | 386 (7.6) | 4670 (92.4) | |
| Female | 127 (10.7) | 1055 (89.3) | |
| Race | 0.526 | ||
| White | 356 (8.4) | 3895 (91.6) | |
| Other | 157 (7.9) | 1830 (92.1) | |
| Marital status | 0.002 | ||
| Single | 125 (8.7) | 1316 (91.3) | |
| Married | 275 (55.7) | 2827 (91.1) | |
| Unknown | 113 (6.7) | 1582 (93.3) | |
| Grade | < 0.001 | ||
| I | 46 (8.8) | 474 (91.2) | |
| II | 148 (17.8) | 684 (82.2) | |
| III | 105 (19.4) | 666 (86.4) | |
| IV | 13 (20.0) | 52 (80.0) | |
| Unknown | 201 (5.0) | 3849 (95.0) | |
| T staging | < 0.001 | ||
| T1 | 172 (11.3) | 1348 (88.7) | |
| T2 | 128 (11.9) | 948 (88.1) | |
| T3 | 154 (5.0) | 2914 (95.0) | |
| T4 | 59 (10.3) | 515 (89.7) | |
| NM staging | < 0.001 | ||
| N0M1 | 212 (6.7) | 2965 (93.3) | |
| N1M0 | 243 (13.7) | 1531 (86.3) | |
| N1M1 | 58 (4.5) | 1229 (95.5) | |
| Tumor size (cm) | < 0.001 | ||
| ≤ 2 | 41 (13.8) | 257 (86.2) | |
| > 2 ≤ 5 | 174 (11.1) | 1398 (88.9) | |
| > 5 | 298 (6.8) | 4070 (93.2) | |
| AFP | < 0.001 | ||
| Negative | 73 (11.4) | 568 (88.6) | |
| Positive | 170 (5.6) | 2872 (94.4) | |
| Unknown | 270 (10.6) | 2285 (89.4) | |
| Fibrosis score | < 0.001 | ||
| 0 - 4 points | 42 (18.1) | 190 (81.9) | |
| 5 - 6 points | 55 (7.3) | 694 (92.7) | |
| Unknown | 416 (7.9) | 4841 (92.1) | |
| Radiation | < 0.001 | ||
| No/unknown | 459 (8.9) | 4699 (91.1) | |
| Yes | 54 (10.2) | 1026 (95.0) | |
| Chemotherapy | 0.329 | ||
| No/unknown | 251 (7.9) | 2930 (92.1) | |
| Yes | 262 (8.6) | 2795 (91.4) |
Clinicopathological Characteristics of Included Patients (N = 6238) a
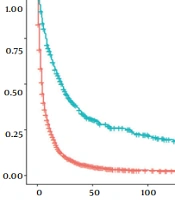
The OS time of 6,238 patients ranged from 1 to 191 months, with a median survival time of 5 months. The median survival time was 20 months for the 513 patients in the surgical group and 4 months in the non-surgical group, with a significantly better median survival time for patients in the surgical group than in the non-surgical group (P < 0.001) (Figure 1). Therefore, this study hypothesized that surgery might be beneficial when the median survival time of patients in the surgery group exceeds 5 months.
4.2. The Patient’s Characteristics After Propensity Score Matching
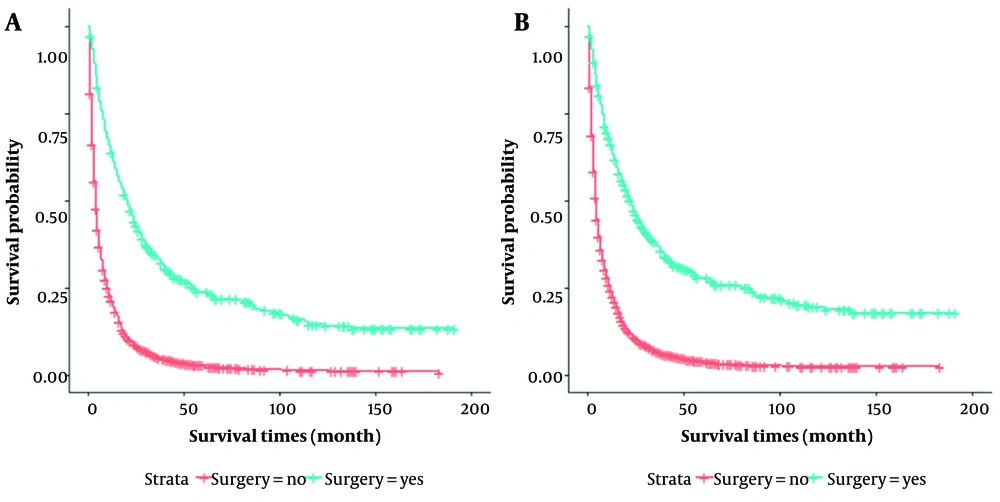
In this study, propensity score matching (PSM) was used for the surgical group (n = 456) versus the non-surgical group (n = 456) to reduce group selection bias. After PSM, as shown in Table 2, all variables were comparable. It was observed that the patients in the surgical and non-surgical groups remained statistically different in OS and CSS (P < 0.001) (Figure 2). The median OS time was 19 months for patients in the surgical group, compared to 4 months for patients in the non-surgical group. The median CSS time was 21 and 5 months for patients in the surgical and non-surgical groups, respectively.
| Variables | Surgical Group (n = 456) | Non-surgical Group (n = 456) | P-Value |
|---|---|---|---|
| Year of diagnosis | 0.314 | ||
| 2004 - 2011 | 258 (48.6) | 273 (51.4) | |
| 2012 - 2019 | 198 (52.0) | 183 (48.0) | |
| Age (y) | 0.389 | ||
| ≤ 60 | 229 (48.6) | 242 (51.4) | |
| > 60 | 227 (51.5) | 214 (48.5) | |
| Gender | 0.528 | ||
| Male | 348 (49.4) | 356 (50.6) | |
| Female | 108 (51.9) | 100 (48.1) | |
| Race | 0.430 | ||
| White | 310 (49.1) | 321 (50.9) | |
| Other | 146 (52.0) | 135 (48.0) | |
| Marital status | 0.560 | ||
| Single | 100 (53.5) | 87 (46.5) | |
| Married | 248 (48.9) | 259 (51.1) | |
| Unknown | 108 (49.5) | 110 (50.5) | |
| Grade | 0.932 | ||
| I | 44 (52.4) | 40 (47.6) | |
| II | 112 (49.6) | 114 (50.4) | |
| III | 91 (48.4) | 97 (51.6) | |
| IV | - | - | |
| Unknown | 209 (50.5) | 205 (49.5) | |
| T staging | 0.095 | ||
| T1 | 145 (51.4) | 137 (48.6) | |
| T2 | 109 (49.8) | 110 (50.2) | |
| T3 | 148 (49.2) | 153 (50.8) | |
| T4 | 54 (49.1) | 56 (50.9) | |
| NM staging | 0.905 | ||
| N0M1 | 199 (49.9) | 200 (50.1) | |
| N1M0 | 199 (50.6) | 194 (49.4) | |
| N1M1 | 58 (48.3) | 62 (51.7) | |
| Tumor size (cm) | 0.541 | ||
| ≤ 2 | 36 (54.5) | 30 (45.5) | |
| > 2 ≤ 5 | 156 (51.5) | 147 (48.5) | |
| > 5 | 264 (48.6) | 279 (51.4) | |
| AFP | 0.369 | ||
| Negative | 58 (50.4) | 57 (49.6) | |
| Positive | 165 (53.1) | 146 (46.9) | |
| Unknown | 233 (47.9) | 253 (52.1) | |
| Fibrosis score | 0.901 | ||
| 0 - 4 points | 26 (51.0) | 25 (49.0) | |
| 5 - 6 points | 53 (52.0) | 49 (48.0) | |
| Unknown | 377 (49.7) | 382 (50.3) | |
| Radiation | 0.598 | ||
| No/unknown | 403 (49.7) | 408 (50.3) | |
| Yes | 53 (52.5) | 48 (47.5) | |
| Chemotherapy | 0.550 | ||
| No/unknown | 217 (51.1) | 208 (48.9) | |
| Yes | 239 (49.1) | 248 (50.9) |
The Characteristics of Patients with Extrahepatic Metastases from Hepatocellular Carcinoma After Propensity Score Matching (N = 912) a
To further explore the factors affecting the survival of patients in the surgical and non-surgical groups, this study adopted COX regression for analysis. It was observed that marital status, grade, T staging, NM staging, tumor size, primary site surgery, AFP, and chemotherapy were factors affecting patients with HCC-EHM both in the univariate analysis and multivariate analysis for OS (P < 0.05), excluding marital status (P = 0.96) (Table 3). In CSS, the multivariate analysis showed that T staging, NM staging, tumor size, primary site surgery, AFP, and chemotherapy remained relevant factors (P < 0.05) (Table 4). Of note was the hepatic fibrosis score in CSS, which showed P = 0.051 and P = 0.038 in univariate analysis and multivariate analysis, respectively (Table 4). Overall survival at 1, 3, and 5 years was 60.7%, 30.3%, and 20.7% in the surgical group, compared to 23.0%, 7.5%, and 3.4% in the non-surgical group, respectively (P < 0.001). Similarly, CSS at 1, 3, and 5 years was significantly higher in the surgical group (63.3%, 34%, and 24.9%) than in the non-surgical group (26.8%, 9.8%, and 4.6%) (P < 0.001).
| Variables | Total (N = 912) | Univariable | Multivariate | ||
|---|---|---|---|---|---|
| P-Value | HR | 95% CI | P-Value | ||
| Year of diagnosis | 0.836 | ||||
| 2004 - 2011 | 531 (58.2) | ||||
| 2012 - 2019 | 381 (41.8) | ||||
| Age (y) | 0.357 | ||||
| ≤ 60 | 471 (51.6) | ||||
| > 60 | 441 (48.4) | ||||
| Gender | 0.505 | ||||
| Male | 704 (77.2) | ||||
| Female | 208 (22.8) | ||||
| Race | 0.196 | ||||
| White | 631 (69.2) | ||||
| Other | 281 (30.8) | ||||
| Marital status | 0.011 | 0.096 | |||
| Single | 187 (20.5) | - | |||
| Married | 507 (55.6) | 0.827 | 0.669 - 1.022 | ||
| Unknown | 218 (23.9) | 1.005 | 0.847 - 1.193 | ||
| Grade | < 0.001 | 0.003 | |||
| I | 84 (9.2) | 0.762 | 0.633 - 0.918 | ||
| II | 226 (24.8) | 0.629 | 0.476 - 0.831 | ||
| III | 188 (20.6) | 0.737 | 0.598 - 0.908 | ||
| IV | - | - | |||
| Unknown | 414 (45.4) | - | |||
| T staging | < 0.001 | < 0.001 | |||
| T1 | 282 (30.9) | 0.706 | 0.554 - 0.899 | ||
| T2 | 219 (24.0) | 0.958 | 0.735 - 1.249 | ||
| T3 | 301 (33.0) | 1.112 | 0.878 - 1.408 | ||
| T4 | 110 (12.1) | - | |||
| NM staging | < 0.001 | < 0.001 | |||
| N0M1 | 399 (43.8) | 0.772 | 0.617 - 0.966 | ||
| N1M0 | 393 (43.1) | 1.140 | 0.915 - 1.421 | ||
| N1M1 | 120 (13.1) | - | |||
| Tumor size (cm) | < 0.001 | 0.001 | |||
| ≤ 2 | 66 (7.2) | 0.495 | 0.362 - 0.677 | ||
| > 2 ≤ 5 | 303 (33.2) | 0.827 | 0.686 - 0.997 | ||
| > 5 | 543 (59.6) | - | |||
| AFP | 0.001 | 0.001 | |||
| Negative | 115 (12.6) | 1.399 | 1.110 - 1.764 | ||
| Positive | 311 (34.1) | 1.581 | 1.241 - 2.014 | ||
| Unknown | 486 (53.3) | - | |||
| Surgery | < 0.001 | < 0.001 | |||
| Yes | 456 (50) | 2.944 | 2.534 -3.421 | ||
| No | 456 (50) | - | |||
| Fibrosis score | 0.109 | ||||
| 0 - 4 points | 51 (5.6) | ||||
| 5 - 6 points | 102 (11.2) | ||||
| Unknown | 759 (83.2) | ||||
| Radiation | 0.271 | ||||
| No/unknown | 811 (88.9) | ||||
| Yes | 101 (11.1) | ||||
| Chemotherapy | 0.002 | < 0.001 | |||
| No/unknown | 425 (46.6) | 1.562 | 1.350 - 1.807 | ||
| Yes | 487 (3.4) | - | |||
Univariate and Multivariate Analyses of Overall Survival in Patients with Extrahepatic Metastases from Hepatocellular Carcinoma a
| Variables | Total (N = 912) | Univariable | Multivariate | ||
|---|---|---|---|---|---|
| P-Value | HR | 95% CI | P-Value | ||
| Year of diagnosis | 0.910 | ||||
| 2004 - 2011 | 531 (58.2) | ||||
| 2012 - 2019 | 381 (41.8) | ||||
| Age (y) | 0.423 | ||||
| ≤ 60 | 471 (51.6) | ||||
| > 60 | 441 (48.4) | ||||
| Gender | 0.451 | ||||
| Male | 704 (77.2) | ||||
| Female | 208 (22.8) | ||||
| Race | 0.123 | ||||
| White | 631 (69.2) | ||||
| Other | 281 (30.8) | ||||
| Marital status | 0.038 | 0.182 | |||
| Single | 187 (20.5) | 0.853 | 0.681 - 1.068 | ||
| Married | 507 (55.6) | 1.021 | 0.851 - 1.225 | ||
| Unknown | 218 (23.9) | - | |||
| Grade | < 0.001 | 0.003 | |||
| I | 84 (9.2) | 0.766 | 0.630 - 0.931 | ||
| II | 226 (24.8) | 0.599 | 0.444 - 0.807 | ||
| III | 188 (20.6) | 0.731 | 0.587 - 0.911 | ||
| IV | - | - | |||
| Unknown | 414 (45.4) | - | |||
| T staging | < 0.001 | < 0.001 | |||
| T1 | 282 (30.9) | 0.679 | 0.527 - 0.875 | ||
| T2 | 219 (24.0) | 0.945 | 0.715 - 1.249 | ||
| T3 | 301 (33.0) | 1.107 | 0.866 - 1.414 | ||
| T4 | 110 (12.1) | - | |||
| NM staging | < 0.001 | < 0.001 | |||
| N0M1 | 399 (43.8) | 0.767 | 0.605 - 0.972 | ||
| N1M0 | 393 (43.1) | 1.178 | 0.934 - 1.485 | ||
| N1M1 | 120 (13.1) | - | |||
| Tumor size (cm) | < 0.001 | < 0.001 | |||
| ≤ 2 | 66 (7.2) | 0.471 | 0.337 - 0.659 | ||
| > 2 ≤ 5 | 303 (33.2) | 0.809 | 0.663 - 0.988 | ||
| > 5 | 543 (59.6) | - | |||
| AFP | < 0.001 | < 0.001 | |||
| Negative | 115 (12.6) | 1.349 | 1.042 - 1.748 | ||
| Positive | 311 (34.1) | 1.681 | 1.296 - 2.179 | ||
| Unknown | 486 (53.3) | - | |||
| Surgery | < 0.001 | < 0.001 | |||
| Yes | 456 (50) | 2.911 | 2.484 - 3.411 | ||
| No | 456 (50) | - | |||
| Fibrosis score | 0.051 | 0.038 | |||
| 0 - 4 points | 51 (5.6) | 0.867 | 0.758 - 0.992 | ||
| 5 - 6 points | 102 (11.2) | - | |||
| Unknown | 759 (83.2) | ||||
| Radiation | 0.095 | ||||
| No/unknown | 811 (88.9) | ||||
| Yes | 101 (11.1) | ||||
| Chemotherapy | 0.011 | < 0.001 | |||
| No/unknown | 425 (46.6) | 1.517 | 1.301 - 1.770 | ||
| Yes | 487 (3.4) | - | |||
Univariate and Multivariate Analyses of Cancer-Specific Survival in Patients with Extrahepatic Metastases from Hepatocellular Carcinoma a
4.3. Nomogram Development and Evaluation
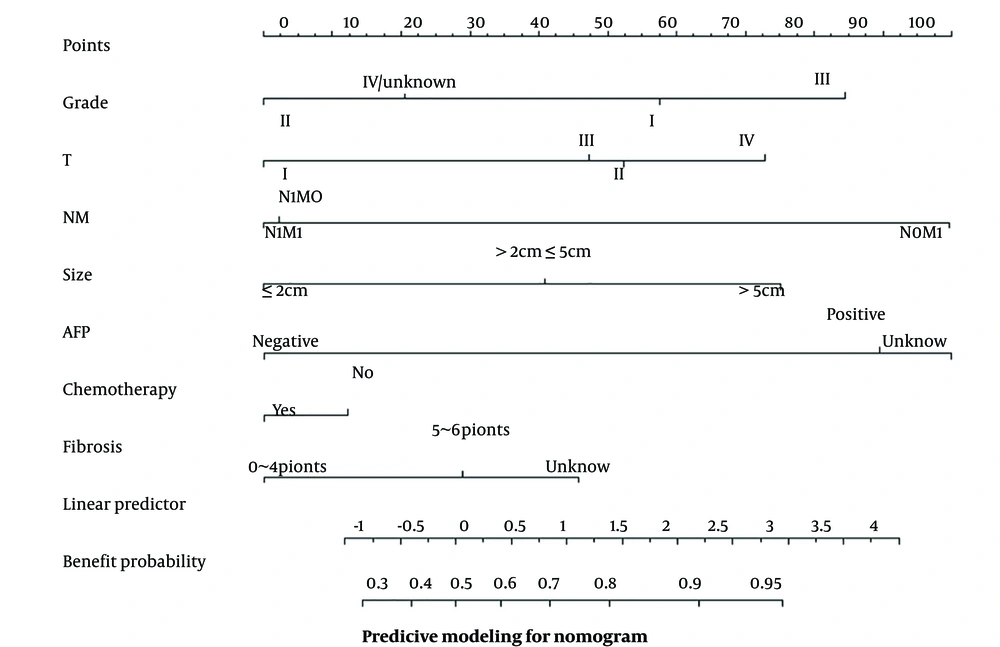
To be able to predict more intuitively the probability of benefit for patients suitable for surgery, this study included seven independent influencing factors (i.e., grade, T staging, NM staging, tumor size, AFP, chemotherapy, and fibrosis score) in the nomogram as a way to determine whether a patient is suitable for surgical treatment. Based on the independent risk factors affecting the prognosis of patients with HCC-EHM, the nomogram can translate each independent risk factor into a specific score (Figure 3). In this study, 456 patients in the surgical group were randomly assigned to the training and validation groups (7:3) to ultimately predict whether patients would benefit from receiving surgical treatment at the primary site.
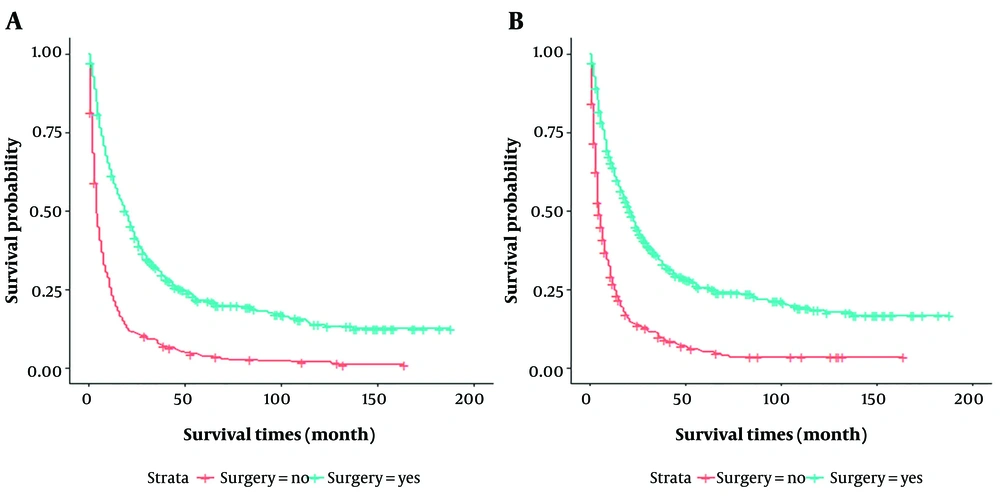
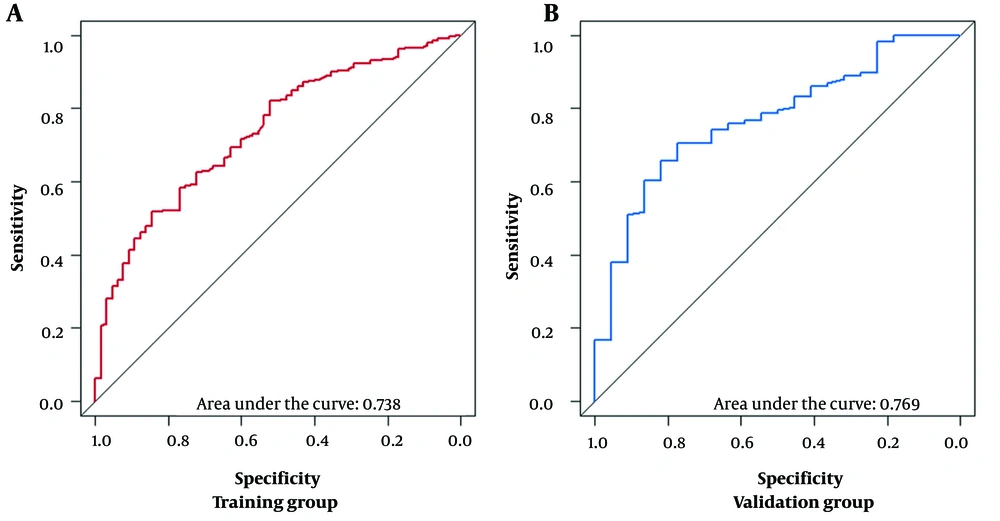
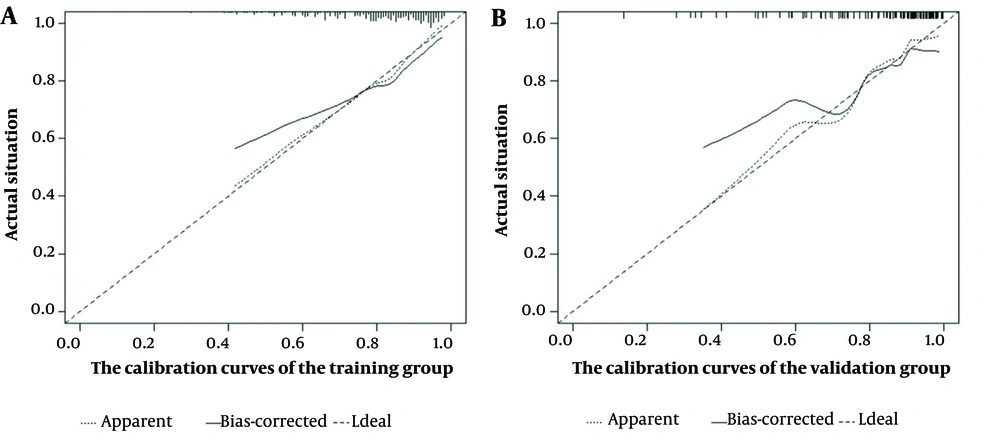
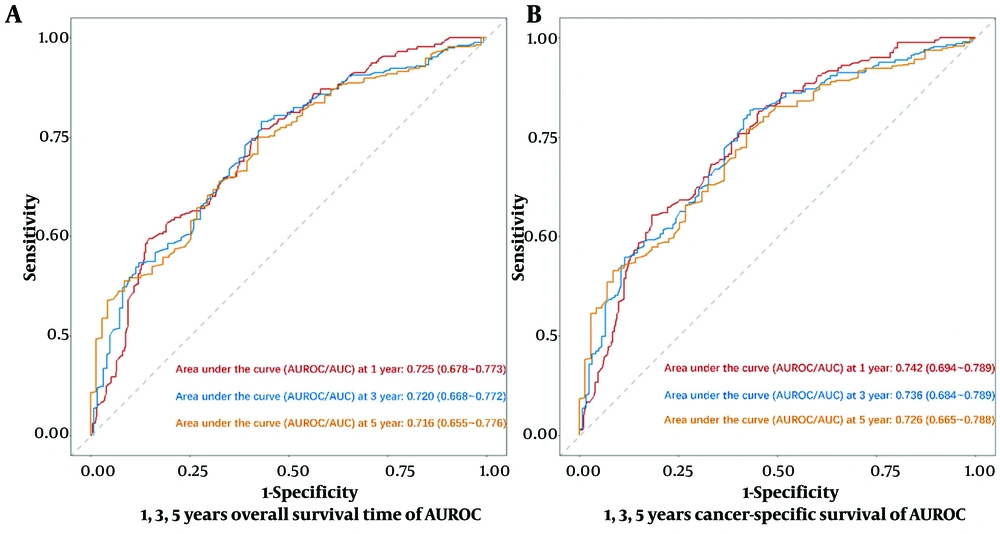
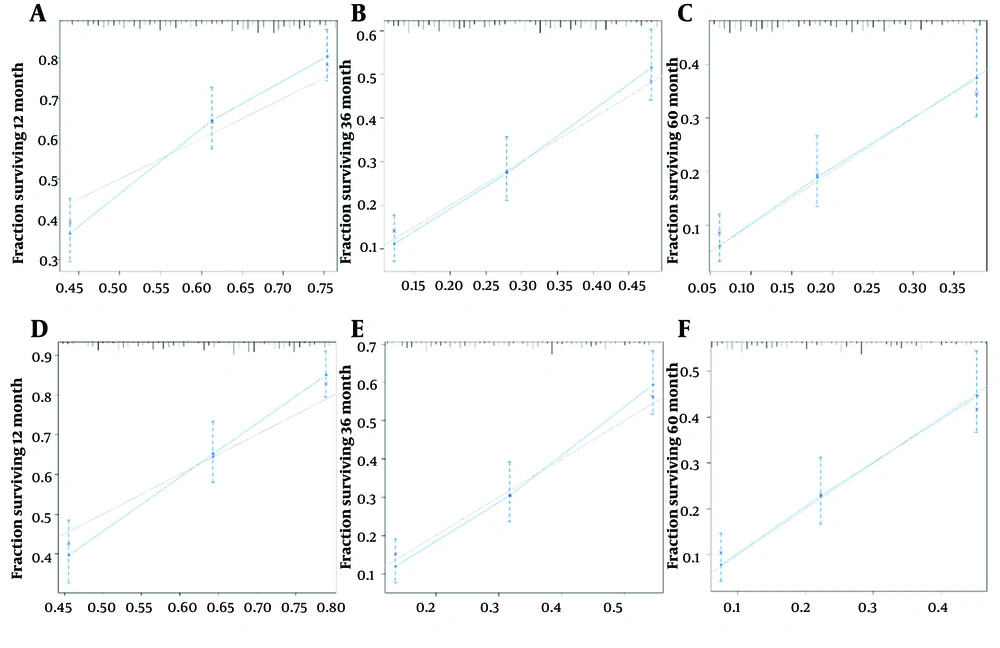
Model calibration curves were plotted using the bootstrap method (B = 1000) with an equal number of put-back replicate samples to further validate the model’s accuracy. The results of ROC curves showed the area under the curve (AUC) of 0.738 and 0.769 for the training and validation groups, respectively (Figure 4). The calibration curves showed that the predictive results of the model correlated well with the actual benefit, both in the training and validation groups (Figure 5). After the present model predictions, the AUCs for patients with 1, 3, and 5 years in OS were 0.725, 0.720, and 0.716. Moreover, the AUCs for patients with 1, 3, and 5 years in CSS were 0.742, 0.736, and 0.726 (Figure 6). By the OS versus CSS calibration curve, it was observed that the present model had a better predictive function (Figure 7).
Decision curve analysis of nomogram for predicting 1, 3, and 5 years of survival in patients with extrahepatic metastatic hepatocellular carcinoma; A, the area under the curve (AUC) of overall survival at 1, 3, and 5 years reported as 0.725 (95% confidence interval (CI): 0.678 - 0.773), 0.720 (95% CI: 0.668 - 0.772), and 0.716 (95% CI: 0.655 - 0.776), respectively; B, the AUC of cancer-specific survival at 1, 3, and 5 years reported as 0.742 (95% CI: 0.694 - 0.789), 0.736 (95% CI: 0.684 - 0.789), and 0.726 (95% CI: 0.665 - 0.788), respectively
Calibration curves of nomogram for 1-, 3-, and 5-year survival probabilities; A, 1 year of survival for overall survival (OS); B, 3 years of survival for OS; C, 5 years of survival for OS; D, 1 year of survival for cancer-specific survival (CSS); E, 3 years of survival for CSS; F, 5 years of survival for CSS
5. Discussion
In this study, the information was collected from the SEER database for 2004 - 2018, and PSM and multiple imputations were used. It was observed that a proportion of HCC-EHM patients still underwent surgery and prolonged their survival time. This suggests that surgery might also be a potentially available treatment option for patients with HCC-EHM. Predicting and evaluating the potential influencing factors for HCC-EHM patients to be able to have surgery will enable further identification of suitable candidates for surgery. The final results showed that the present model could screen patients with HCC-EHM who were more suitable for surgical treatment. In addition, the current nomogram can predict the survival of such patients with good predictive power in both OS and CSS.
For patients with advanced HCC, systemic targeted immunotherapy, chemotherapy, radiotherapy, and other symptomatic supportive therapy are the recommended main treatment modalities. However, some patients are still treated surgically in previous publications. The development of surgical treatment techniques, with modern surgery that is more precise, less invasive, with faster recovery, might likewise extend the survival time of patients. Currently, in patients with HCC, partial hepatectomy is mainly indicated in good physical condition, with good liver function (child-Pugh A/B), adequate residual volume of the liver, and no significant vascular invasion (11).
Some studies have shown that patients with advanced HCC who undergo surgery alone or in combination with other treatments can achieve good results (12, 13). Another study concluded that HCC-EHM patients could achieve a better survival time than other treatment modalities by modestly expanding the resection criteria (14). Numerous previous studies have also shown that surgical treatment, TACE, RFA, and radiation therapy for the primary tumor in the liver can still improve the long-term survival time of patients with HCC-EHM (10, 15, 16). One study showed that patients treated with surgical resection had a longer survival time than those treated with thermal injection ablation (17). It has been suggested that some patients with advanced HCC might be potentially eligible for surgery; however, there is a lack of objective evaluation indicators (18).
Conversion therapy converts an otherwise inoperable advanced tumor into an operable tumor through systemic or local treatment (18). Using Lenvatinib plus anti-PD-1 antibodies in patients with HCC-EHM might allow downstaging and subsequent eligibility for surgical resection in a proportion of patients with advanced HCC (19). However, another study concluded that in patients with HCC-EHM, the effectiveness of surgical treatment at the primary site is unclear (20). The suitability of surgical treatment for patients with HCC-EHM still needs to be confirmed in additional prospective studies. Although surgical treatment might decrease liver function and immunity in patients, it still increases overall long-term survival (21, 22).
Not only for HCC patients but also in patients with advanced ovarian, kidney, and colorectal cancers, primary site surgery has been performed with promising results (23-26). All these studies might indicate that even if the guidelines do not recommend surgical treatment, the survival time of patients can still be prolonged by surgery. The beneficial effect of surgery was also confirmed in the current study.
In the present newly designed model, grading, staging, AFP, and size were well-known predictors for the prognosis of HCC patients. Age was not an independent risk factor; however, one study considered lower age (< 60 years) as an independent risk factor, which might be related to the early date of the survey and the small number of patients (n = 32) (27). Mao et al. concluded that age > 80 years, tumor size > 10 cm, TNM stage, and vascular invasion were associated with OS and CSS in patients with HCC-EHM and did not analyze patients’ chemotherapy and radiotherapy (9). Similarly, age, T stage, tumor size, radiotherapy, and chemotherapy were correlated with OS and CSS by Chen et al. Nevertheless, in the present study, age was not an independent risk factor (12). It is believed that the reason for this might be that these studies only included the data from the pre-2015 SEER database. With the development and advancement of surgical techniques, older patients are at significantly lower risk of undergoing surgical treatment than before.
In the current study, although the hepatic fibrosis score was not statistically significant in the univariate analysis (P = 0.051), it was still included in the multivariate analysis, after which the fibrosis score reflected a statistically significant difference (P = 0.038). The hepatic fibrosis score was not populated in the early SEER database and has only been refined in recent years. The present analysis suggests that the hepatic fibrosis score is a potential predictor of OS and CSS in patients with HCC-EHM. Previous studies have suggested the significance of liver fibrosis for long-term survival in HCC patients, which is the same as the current study (28). The role of chemotherapeutic drugs combined with targeted drugs is indispensable for the treatment of advanced HCC, and it is not impossible to try to use multiple therapies in combination, including surgery, in order to eventually obtain an extended survival time for patients.
The current study has several advantages. Firstly, the SEER database used in this study is a population-based study, not a single-center study. Secondly, PSM was used to minimize selection biases. Thirdly, this study performed internal data validation, demonstrating the model’s good ability. However, this study still has some limitations. The nomogram has fewer variables, and patients should be assessed more individually. Secondly, this study was retrospective, and other potential predictors were unavailable in the SEER database (e.g., immunotherapy and targeted drug therapy). In addition, it is suggested to use further prospective clinical studies for the systematic assessment of the model accuracy.