1. Background
Due to the often asymptomatic and subclinical nature of chronic viral hepatitis infections, a significant number of individuals with hepatitis B virus (HBV) or hepatitis C virus (HCV) remain unaware of their condition (1, 2). As a result, screening programs for HBV and HCV are crucial for identifying infected individuals, facilitating their access to care, and initiating effective treatments to mitigate the risk of liver disease and mortality. Although the overall prevalence of HBV and HCV in the Netherlands is relatively low, at 0.34% and 0.16%, respectively, in 2016 (3), the majority of chronic HBV or HCV cases in the country are among migrants from regions where these viruses are endemic. In these migrant groups, the estimated prevalence rates are 3.1% for HBV and 0.9% for HCV (3). However, these individuals often remain untested by programs aimed at eliminating viral hepatitis (3, 4). Thus, a primary goal of the Dutch national hepatitis strategy is to identify and diagnose HBV and HCV among migrants (4).
A recent retrospective study within various ethnic groups in Amsterdam revealed a notably high prevalence of HBV, especially among individuals of Ghanaian and Turkish descent (5), while HCV prevalence was lower than anticipated based on their countries of origin (5). This study randomly selected individuals from ethnic minority groups who were born abroad (first-generation migrants) without considering specific risk factors for viral hepatitis. Given the particularly low prevalence of HCV, a more targeted screening approach might yield greater efficiency.
One proposed method for targeted screening focuses on testing individuals with elevated transaminase levels or other conditions linked to viral hepatitis. The Dutch national hepatitis plan highlights the underutilization of viral hepatitis testing by healthcare professionals in individuals with elevated alanine aminotransferase (ALT) levels as a significant concern (4). Hepatic steatosis is common among those with chronic HBV and/or HCV, affecting about 30% of individuals with HBV and up to 86% of those with HCV genotype 3 (6, 7). Furthermore, both HBV and HCV are linked to insulin resistance and a higher prevalence of type 2 diabetes mellitus (T2DM) (8), making hepatic steatosis and T2DM potential indicators for chronic viral hepatitis.
2. Objectives
Our study aimed to evaluate the prevalence of chronic HBV and HCV among individuals from various ethnic groups who have (1) elevated transaminases or (2) metabolic risk factors and to compare the screening efficiency of targeting these two groups to a generic screening approach.
3. Methods
3.1. Study Design and Population
This study is a component of the multi-ethnic perspective HELIUS study (Healthy Life in an Urban Setting), which seeks to understand the unequal distribution of disease and its determinants across different ethnic groups. HELIUS encompasses 24 782 participants from six major ethnic groups living in Amsterdam, the Netherlands, including Dutch, African Surinamese, South-Asian Surinamese, Turkish, Moroccan, and Ghanaian origins. Further details on the migration history of these groups are available in previous publications (9, 10). Briefly, these represent the largest migrant populations in Amsterdam (i.e., Surinamese, Turkish, and Moroccan), with similar groups also present in other European countries. Individuals aged between 18 and 70 were randomly selected from the municipality register of Amsterdam, with stratification by ethnicity. Additional information on the design and selection procedures of the HELIUS study is available in other sources (9, 10).
Baseline measurements were conducted between 2011 and 2015. Subsequently, five hundred individuals of Dutch origin and 2 500 first-generation migrants (500 each from Turkish, Moroccan, and Ghanaian backgrounds, and 1 000 of Surinamese origin) were randomly chosen for a hepatitis B and C sub-study. Of these, 2 993 were retrospectively tested for anti-HCV and 2 987 for anti-HBc. More information on this data collection is described in a previous publication (5).
Of the 24 782 initial participants, 290 had passed away, and 498 had relocated abroad before the commencement of the follow-up measurement phase. Consequently, 23 994 participants were invited to attend the first follow-up measurement between 2019 and 2022, a phase that was still ongoing at the time of this research. By the end of 2021, 10 585 participants had completed their follow-up visit. Concurrently, a sub-study focusing on non-alcoholic fatty liver disease (NAFLD), titled "NAFLD in the Healthy Life in an Urban Setting cohort" (NILE study), was conducted (11). The objective was to determine the prevalence of NAFLD across various ethnic groups in Amsterdam, the Netherlands. For the NILE study, individuals were randomly chosen from the follow-up participants of the HELIUS study, stratifying by age, sex, and ethnicity based on data collected during the follow-up visit. More comprehensive information regarding the NILE selection procedure is available in other documents (11).
In summary, eligibility for NILE was determined based on the presence of either an elevated non-invasive liver test (NIT) or a metabolic risk factor. The NITs encompassed the AST to platelet ratio index (APRI), fibrosis-4 index (FIB-4), and fatty liver index (FLI), developed to predict hepatic fibrosis and steatosis (12, 13). Specifically, a FIB-4 cut-off of ≥ 1.3 and an APRI cut-off of ≥ 0.42 were indicative of potential hepatic fibrosis, while a FLI cut-off of ≥ 30 suggested potential hepatic steatosis (14). Metabolic risk factors were identified as type T2DM, obesity (BMI ≥ 30 kg/m2), and an elevated waist-hip ratio (WHR > 0.90 m for men and > 0.85 m for women). Given the primary goal of the NILE study to identify NAFLD cases, individuals consuming more than 21 units of alcohol weekly were excluded, as were those without an available blood sample. The study did not filter participants based on their liver disease history. Moreover, a control group randomly selected from all six ethnicities, without elevated NITs or metabolic risk factors, was also invited to participate (11).
Eligible individuals were invited to participate in the NILE study visit, which involved a liver stiffness measurement using Fibroscan® (Echosens, Paris, France) and additional blood sampling. The blood samples collected during the NILE study were initially tested for antibodies against the hepatitis B core (anti-HBc) and HCV (anti-HCV). If positive, the samples were further analyzed for hepatitis B serum antigen (HBsAg) and/or HCV-RNA, as applicable. Hepatitis B virus and HCV-related serology results were obtained using Liaison XL (DiaSorin, Saluggia, Italy), boasting a sensitivity and specificity above 99.7% (15). For determining the HCV viral load, the Alinity system (Abbott, Abbott Park, Illinois, United States) was utilized, offering a detection rate of over 98% for samples with an HCV RNA viral load near the lower detection limit (i.e., 5.11 IU/mL) (16). HBsAg-positive samples underwent additional testing for anti-hepatitis D virus antibodies (anti-HDV). All participants of the NILE study with available HBV and HCV serology results were included in this sub-study.
Demographic information, such as age, sex, ethnic background, and migration generation, was gathered during the HELIUS baseline. Laboratory results (AST, ALT, platelet count) and cardiovascular risk factors (BMI, waist-to-hip ratio, presence of diabetes mellitus) were collected during the HELIUS follow-up, along with self-reported alcohol consumption. Additionally, self-reported risk factors for viral hepatitis (previous injecting drug use, blood transfusion, history of surgery, belonging to the men who have sex with men key population) were collected during the HELIUS NILE study. More detailed information on the collection and classification of demographic variables is available in the supplementary data. Participants were also asked to bring their prescribed medications to the physical examination, which were coded according to the Anatomical Therapeutic Chemical (ATC) classification system, including medication for addiction (ATC code N07B). Type 2 diabetes mellitus status was determined based on self-reported diagnosis and/or the use of T2DM-related medication. The most recently available data were used for all mentioned variables.
3.2. Statistical Analysis
For statistical analysis, descriptive statistics were employed to summarize participant characteristics and the outcomes of viral hepatitis testing. The chi-square test or Mann-Whitney U test was used to compare participant characteristics. Hepatitis B virus and HCV testing results were reported for both the overall study population and by ethnic group.
We identified two different targeted screening strategies: (1) Testing all individuals in the NILE study for potential liver fibrosis according to NITs (as indicated by the APRI or FIB-4 index); and (2) testing individuals with a metabolic risk factor (i.e., T2DM, obesity, elevated WHR, or meeting FLI criteria). A generic screening approach was also defined, involving general testing (i.e., including individuals from the randomly selected control group in the NILE study and participants from a previously conducted, retrospective HBV/HCV study during the first HELIUS cohort visit), without selecting based on a priori risk of liver disease, except for including only first-generation migrants (5).
We evaluated the effectiveness of each screening strategy, which is defined as the ratio of positive tests to the total number of individuals tested. Additionally, we calculated the sensitivity and specificity of each approach as further measures of diagnostic accuracy. The analysis was further stratified by the endemicity of HBV infection based on the HBsAg-prevalence from a previous HBV study in the HELIUS cohort (5). We classified HBV endemicity into three groups: Dutch-origin groups, non-Dutch ethnic groups with a low-endemic HBV status (HBsAg-prevalence < 2%, including Moroccan and South-Asian Surinamese participants) (5, 17), and non-Dutch ethnic groups with an intermediate-endemic HBV status (HBsAg-prevalence 2 - 8%, including Turkish, Ghanaian, and African Surinamese participants). A sensitivity analysis was also conducted, focusing solely on first-generation migrants. Due to the low number of anti-HCV positive individuals, we opted not to conduct analyses on HCV testing results after considering it further. R software (version 4.0.3, Vienna, Austria) was used for data analysis.
4. Results
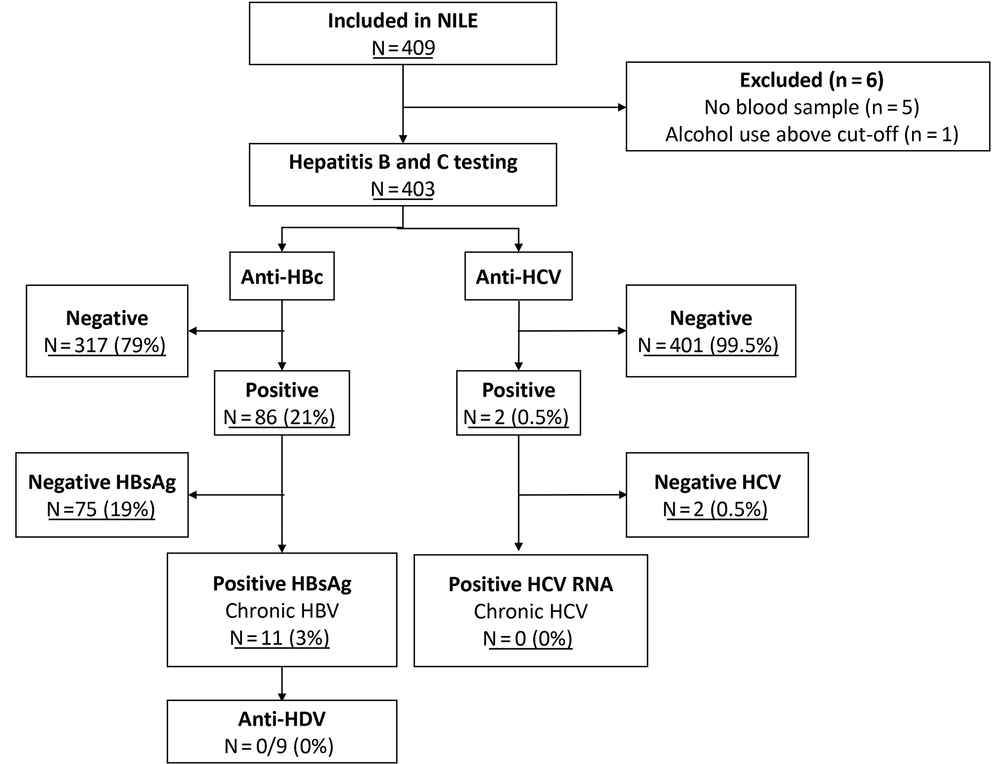
Results highlighted that of the 10 585 participants in the HELIUS follow-up, 2 960 (30%) exhibited an APRI and/or FIB-4 value indicating hepatic fibrosis, while 7 792 (78%) met at least one metabolic risk factor criterion. A total of 655 individuals were invited to the NILE study, with 409 (62%) agreeing to participate and completing their NILE study visit. Six additional individuals were excluded due to missing blood samples (n = 5) or exceeding the alcohol use criterion (n = 1), resulting in 403 participants being included in the analysis.
Among these, the majority were of Dutch origin (n = 103, 26%), followed by South-Asian Surinamese (n = 91, 23%), African Surinamese (n = 89, 22%), and Ghanaian (n = 63, 16%). These participants' characteristics are detailed in Table 1. APRI or FIB-4 values above the hepatic fibrosis cut-off were found in 218 (54%) individuals, 323 (80%) had at least one metabolic risk factor, and 53 (13%) were included in the control group. In total, 192 (48%) individuals qualified under both the NIT and metabolic risk factor categories.
| Variables | Dutch (n = 103) | African Surinamese (n = 89) | South-Asian Surinamese (n = 91) | Ghanaian (n = 63) | Moroccan (n = 36) | Turkish (n = 21) | Total (n = 403) |
|---|---|---|---|---|---|---|---|
| Female sex | 50 (49) | 43 (48) | 44 (48) | 29 (46) | 16 (44) | 11 (52) | 193 (48) |
| Age (median, IQR) | 58 (49 - 70) | 59 (48 -70) | 60 (48 - 68) | 58 (51 - 63) | 53 (45 - 69) | 48 (40 - 56) | 58 (48 - 67) |
| First-generation migrant | Not applicable | 77 (87) | 76 (84) | 62 (98) | 28 (78) | 11 (52) | 254/300 (85) |
| Viral hepatitis risk factors | 12 (12) | 20 (24) | 11 (14) | 7 (13) | 4 (13) | 2 (10) | 56 (14) |
| Prior injecting drug use | 0 | 1 (1) | 1 (1) | 0 | 0 | 0 | 2 (0.5) |
| Blood transfusion < 1992, HIC | 5 (5) | 4 (4) | 1 (1) | 0 | 1 (3) | 0 | 11 (3) |
| Blood transfusion, LMIC | 0 | 4 (4) | 5 (5) | 3 (5) | 0 | 0 | 12 (3) |
| Surgery, LMIC | 1 (1) | 11 (12) | 5 (5) | 6 (10) | 3 (8) | 2 (10) | 28 (7) |
| Men who had sex with men | 4 (4) | 1 (1) | 1 (1) | 0 | 1 (3) | 0 | 7 (2) |
| > 1 year in a household with HCV-positive individual or IDU | 2 (2) | 2 (2) | 1 (1) | 0 | 1 (3) | 0 | 6 (1) |
| Use of medication for addiction b | 0 | 1 (1) | 0 | 0 | 0 | 0 | 1 (0.2) |
| Non-invasive tests | |||||||
| FLI ≥ 60 | 55 (53) | 36 (41) | 35 (56) | 21 (58) | 53 (58) | 12 (57) | 212 (53) |
| FIB-4 ≥ 1.30 | 45 (44) | 42 (48) | 27 (43) | 24 (71) | 42 (46) | 19 (91) | 199 (49) |
| APRI ≥ 0.42 | 30 (29) | 24 (27) | 23 (37) | 2 (6) | 31 (34) | 1 (5) | 111 (28) |
| Metabolic risk factors | |||||||
| Body mass index | 28 (24 - 32) | 31 (27 - 34) 13 | 28 (24 - 31) | 29 (27 - 32) | 29 (26 - 32) | 27 (24 - 35) | 29 (25 - 32) |
| Type 2 diabetes mellitus | 13 (13) | (15) | 16 (18) | 17 (27) | 9 (25) | 2 (10) | 70 (17) |
| Waist-hip ratio ≥ 0.90 | 76 (75) | 72 (81) | 77 (85) | 57 (92) | 32 (89) | 16 (76) | 330 (83) |
| Liver stiffness measurement | |||||||
| < 7.0 kPa | 82 (80) | 73 (82) | 73 (80) | 59 (94) | 29 (81) | 16 (76) | 332 (82) |
| ≥ 7.0 kPa - < 9.5 kPa | 15 (15) | 14 (16) | 10 (11) | 2 (3) | 3 (8) | 3 (14) | 47 (12) |
| ≥ 9.5 kPa - < 12.5 kPa | 5 (5) | 2 (2) | 1 (1) | 2 (3) | 3 (8) | 0 | 13 (3) |
| ≥ 12.5 kPa | 1 (1) | 0 | 7 (8) | 0 (0) | 1 (3) | 2 (10) | 11 (3) |
| CAP (median, IQR) | 268 (220 - 319) | 258 (219 - 298) | 279 (244 - 322) | 243 (218 - 277) | 255 (205 - 297) | 262 (219 - 306) | 260 (222 - 307) |
| CAP ≥ 280 dB/m | 47 (46) | 30 (34) | 45 (50) | 15 (24) | 12 (33) | 7 (33) | 156 (39) |
Abbreviations: IQR, interquartile range; HCV, hepatitis C virus; HIC, high-income country; LMIC, lower- and middle-income country; IDU, injecting drug user; APRI, AST to platelet ratio index; FIB-4, fibrosis-4 index; FLI, fatty liver index; CAP, controlled attenuation parameter; kPa, kiloPascal; dB/m, decibel/meter.
a Values are expressed as median (percentage) unless otherwise noted.
b Medication for addiction based on ATC code N07B.
Characteristics of the 246 individuals invited to the NILE study who did not attend are detailed in Appendix 1. Compared to non-participants, included participants were younger (median age 57 vs. 59, P = 0.02), more often of Dutch origin (26% vs. 14%, P < 0.01), and among migrants, were less frequently first-generation (85% vs. 97%, P < 0.01), without significant differences in gender (48% female in both groups, P = 0.36) or self-reported risk factors for viral hepatitis (14% in both groups, P = 1.00).
Hepatitis B virus and HCV test results are summarized in Figure 1. Regarding HBV, 317 (79%) individuals tested negative for anti-HBc, 75 (19%) were anti-HBc positive and HBsAg negative, and 11 (3%) were HBsAg positive. Among the 11 HBV-infected individuals, eight (73%) were previously unaware of their HBV status, while three were known cases not currently engaged in HBV care. One individual with HBsAg positivity, unaware of their HBV status, exhibited a liver stiffness measurement indicative of advanced fibrosis or cirrhosis (23 kPa). Anti-HDV testing was performed for 9 of the 10 HBsAg-positive individuals, all of whom tested negative. One sample lacked sufficient volume for the anti-HDV test. Regarding HCV, two participants of Ghanaian origin were found anti-HCV positive but HCV RNA negative, indicating cleared HCV infections. Neither was previously treated for HCV nor aware of their infection status, and both reported no known HCV-related risk factors.
The highest prevalence of anti-HBc positivity was observed among participants of Ghanaian origin (40 out of 63, 64%, 95%CI: 51 - 75%), followed by African Surinamese (24 out of 89, 27%, 95%CI: 19 - 37%), and Turkish origin (4 out of 21, 19%, 95%CI: 7 - 39%) (Table 2). The highest HBsAg positivity rates were in the Ghanaian (4 out of 63, 6%, 95%CI: 2 - 14%), Moroccan (2 out of 36, 6%, 95%CI: 1 - 17%), and Turkish (1 out of 21, 5%, 95%CI: 0.5 - 20%) groups. None of the Dutch-origin participants tested HBsAg positive, in contrast to 4 out of 127 (3%, 95%CI: 8 - 20%) and 7 out of 173 (4%, 95%CI: 2 - 8%) from non-Dutch groups with low and intermediate HBV endemicity, respectively.
| Variables | No. | HBV Serology Results | |||
|---|---|---|---|---|---|
| Anti-HBc-Positive | HBsAg-Positive | ||||
| No. (%) | 95% CI | No. (%) | 95% CI | ||
| Dutch | 103 | 1 (1) | 0.1 - 4 | 0 | 0 - 2 |
| HBsAg low-endemic group | 127 | 17 (13) | 8 - 20 | 4 (3) | 1 - 7 |
| Moroccan | 36 | 6 (17) | 7 - 31 | 2 (6) | 1 - 17 |
| South-Asian Surinamese | 91 | 11 (12) | 7 - 20 | 2 (2) | 0.5 - 7 |
| HBsAg intermediate-endemic group | 173 | 68 (39) | 32 - 47 | 7 (4) | 2 - 8 |
| Ghanaian | 63 | 40 (64) | 51 - 75 | 4 (6) | 2 - 14 |
| African Surinamese | 89 | 24 (27) | 19 - 37 | 2 (2) | 0.5 - 7 |
| Turkish | 21 | 4 (19) | 7 - 39 | 1 (5) | 0.5 – 20 |
Abbreviations: HBV, hepatitis B virus; Anti-HBc, hepatitis B core antibodies; HBsAg, hepatitis B surface antigen; CI, confidence interval.
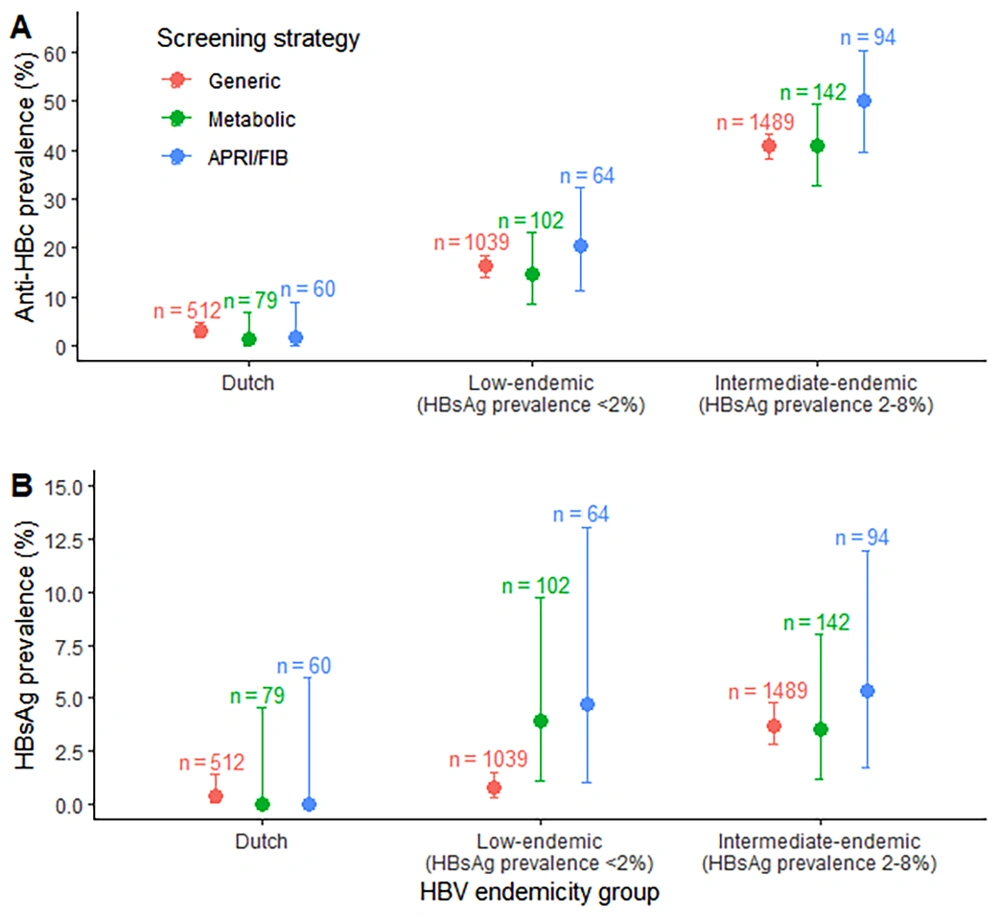
Hepatitis B virus testing outcomes by screening approach are detailed in Table 3 and illustrated in Figure 2. For individuals of Dutch origin, HBsAg prevalence was low across all screening strategies (< 0.4%). In contrast, among participants from non-Dutch groups with intermediate HBV endemicity, HBsAg prevalence exceeded 3.5% across all strategies. In non-Dutch groups with low HBV endemicity, HBsAg prevalence was 0.8% (95%CI: 0.3 - 1.5%) in the generic screening group, 3.9% (95%CI: 1.1 - 9.7%) in those screened for metabolic risk factors, and 4.7% (95%CI: 1.0 - 13.1%) in those screened for elevated liver NITs.
| Variables | No. | HBsAg Serology Results | |
|---|---|---|---|
| HBsAg-Positive a | 95% CI | ||
| Dutch | |||
| Generic screening | 512 | 2 (0.4) | 0.05 – 1.4 |
| Elevated liver fibrosis NITs | 60 | 0 (0) | 0 – 6.0 |
| Metabolic risk factors | 79 | 0 (0) | 0 – 4.6 |
| HBsAg low-endemic group b | |||
| Generic screening | 1039 | 8 (0.8) | 0.3 – 1.5 |
| Elevated liver fibrosis NITs | 64 | 3 (4.7) | 1.0 – 13.1 |
| Metabolic risk factors | 102 | 4 (3.9) | 1.1 – 9.7 |
| HBsAg intermediate-endemic group c | |||
| Generic screening | 1489 | 55 (3.7) | 2.8 – 4.8 |
| Elevated liver fibrosis NITs | 94 | 5 (5.3) | 1.7 – 12.0 |
| Metabolic risk factors | 142 | 5 (3.5) | 1.2 – 8.0 |
Abbreviations: HBsAg, hepatitis B surface antigen; NITs, non-invasive tests.
a Values are expressed as No. (%).
b The low-endemic HBV group included participants with a Moroccan or South-Asian Surinamese ethnic background.
c The intermediate-endemic HBV group included participants with a Ghanaian, Turkish, or African Surinamese ethnic background.
Hepatitis B virus serology testing results were based on different screening approaches in the NILE study, stratified by HBV endemicity in the respective population in Amsterdam, the Netherlands. The low-endemic HBV group included participants with a Moroccan or South-Asian Surinamese ethnic background. The intermediate-endemic HBV group included participants with a Ghanaian, Turkish, or African Surinamese ethnic background. A, anti-HBc testing results; B, HBsAg testing results. Abbreviations: APRI, AST to platelet ratio index; FIB, Fibrosis-4 Index for liver fibrosis; HBV, hepatitis B virus; Anti-HBc, hepatitis B core antibodies; HBsAg, hepatitis B surface antigen.
Regarding the metabolic risk factor screening approach, sensitivity was 100% (95%CI: 40 - 100%) for the low-endemic HBV group and 71% (95%CI: 29 - 96%) for the intermediate-endemic HBV group. The specificity was 20% (95%CI: 14 - 29%) and 22% (95%CI: 16 - 29%) for these respective groups. For the NIT screening approach, sensitivity was 75% (95%CI: 19 - 99%) for the low-endemic HBV group and 71% (95%CI: 29 - 96%) for the intermediate-endemic HBV group, with specificities of 50% (95%CI: 41 - 60%) and 46% (95%CI: 38 - 54%), respectively.
In total, 9 out of 254 (3.5%) first-generation migrants and 2 out of 46 (4.3%) second-generation migrants were HBsAg-positive. Second-generation migrants who were HBsAg-positive included one participant of South-Asian Surinamese and one of Turkish origin. The sensitivity analysis, which included only first-generation migrants, yielded results similar to the main analysis (Appendix 2). For participants in the intermediate HBV endemicity group, HBsAg-prevalence exceeded 3.6% across all three screening strategies. For the low HBV endemicity group, HBsAg-prevalence was 0.8% (95%CI: 0.3 - 1.5%) for the generic screening strategy, 3.4% (95%CI: 0.7 - 9.6%) for the group targeted for metabolic risk factors, and 3.4% (95%CI: 0.4 - 11.7%) for the group targeted for elevated liver NITs.
5. Discussion
Identifying individuals with undiagnosed HBV or HCV among migrant populations is critical for the elimination of chronic viral hepatitis in the Netherlands. This population-based, multi-ethnic study confirmed the low HCV prevalence and high HBV prevalence among various non-Dutch ethnic groups in Amsterdam, the Netherlands (5). Moreover, we evaluated the efficiency of screening when targeting individuals with liver fibrosis as indicated by NITs or metabolic risk factors compared to using a generic screening approach.
No statistically significant differences emerged between the various HBV screening strategies within the overall study cohort, likely due to the small size of the groups targeted for screening. However, our analysis did reveal some notable insights. For individuals from intermediate HBV endemic regions, targeted screening may not be advisable, as the prevalence of HBsAg observed across all groups suggests that a generic screening approach for these individuals is warranted. Conversely, for those from areas with a low HBV endemicity (e.g., Moroccan or South-Asian Surinamese populations), our findings suggest that screening based on metabolic risk factors or liver NITs could be a more efficient method for HBV screening. Clearly, larger-scale studies with adequate sample sizes are necessary to validate these findings.
A modeling study assessing the cost-effectiveness of screening first-generation migrants for HBV in the Netherlands concluded that screening individuals from countries with an HBsAg-prevalence of at least 0.41% would be cost-effective (18). This supports the potential for a generic screening strategy for migrants from low-endemic countries, given that the HBsAg-prevalence in this group was 0.8%. Nevertheless, there are several arguments in favor of adopting a targeted screening approach for this cohort. Firstly, targeted screening could enhance the efficiency of HBV screening, potentially improving the cost-benefit ratio. Secondly, screening individuals with metabolic risk factors might be more practically implemented in clinical settings than generic screening, as it could be incorporated into existing cardiovascular risk assessments. Investigating the accessibility of metabolic risk factor data in primary care electronic health records could offer insights into the practicality of this screening strategy. For NAFLD, primary care screening programs have already proven effective in enhancing the detection rates of advanced fibrosis and cirrhosis and in reducing unnecessary referrals (12). Thirdly, the majority of HBsAg-positive individuals in our study were unaware of their infection, underscoring that current HBV screening efforts in these populations are inadequate despite the demonstrated cost-effectiveness of universal HBV screening.
The prevalence of NAFLD is on the rise, concurrently increasing the co-occurrence of NAFLD with chronic HBV, which necessitates an examination of their potential interplay (19-21). Both hepatic steatosis and T2DM are linked with a quicker progression of liver fibrosis and, consequently, a higher incidence of hepatocellular carcinoma (HCC) in those with chronic viral hepatitis (22, 23). Furthermore, studies involving both treatment-naïve and treatment-experienced individuals with chronic HBV have indicated that those with concurrent NASH often had advanced fibrosis and a reduced timeframe for developing liver-related outcomes compared to those with chronic HBV alone (24). This emphasizes the significance of HBV testing and treatment in individuals with NAFLD or metabolic risk factors, aiming to facilitate early diagnosis and connection to care for those undiagnosed with HBV, ensuring they receive appropriate treatment before the onset of liver-related complications.
In our study, no individuals with detectable HCV RNA were identified. This finding aligns with prior research indicating that chronic HCV infection was observed exclusively in participants of African-Surinamese and Dutch descent in Amsterdam, each group showing a prevalence of 0.4% (5). Notably, two Ghanaian participants were found to have resolved HCV infection, neither of whom had undergone treatment or were aware of their infection previously.
This study has several limitations. Firstly, HBV and HCV testing outcomes were secondary objectives of the NILE study, leading to a relatively small participant count. This resulted in broad confidence intervals and limited our ability to draw definitive conclusions on screening efficiency. Additionally, there might be selection bias due to a loss of follow-up between the first and second HELIUS studies and from HELIUS-2 to the NILE study. The participation rate for the NILE study was 62%, with notably lower participation among individuals of Turkish or Moroccan descent. Nonetheless, the proportion of individuals reporting at least one risk factor for viral hepatitis was consistent at 14% among both participants and those invited but not participating. Participants consuming more than 21 units of alcohol weekly were excluded because the primary focus of the NILE study was on NAFLD screening, which cannot be accurately diagnosed with significant alcohol use. Excluding individuals with heavy alcohol use might inadvertently omit those at an increased risk for viral hepatitis due to overlapping risk factors like substance abuse. HIV data was not available for this study. Moreover, the study largely took place during the COVID-19 pandemic, which likely affected the response rate and reduced the sample size. Lastly, only descriptive statistics were utilized to compare the diagnostic efficiency of targeted versus generic screening approaches because the small number of individuals testing positive for HBsAg hindered any multivariable analyses of these results.
In conclusion, despite the limitations posed by the small sample size, this study indicates a high HBsAg-prevalence among individuals of non-Dutch origin within this multi-ethnic cohort, primarily composed of individuals at risk for NAFLD. Therefore, HBV screening is recommended for all individuals of Ghanaian, African, Surinamese, or Turkish origin. However, for those of Moroccan or South-Asian Surinamese descent, a targeted screening approach focusing on metabolic risk factors or liver fibrosis indicated by NITs might be more advantageous.