1. Background
Over the past decade, immune checkpoint inhibitors (ICIs) have been shown to be effective in the treatment of many malignancies, and their use is on the rise (1, 2). Immunosuppressive molecules are expressed in immune cells, and these can inhibit the immune system and prevent the body from forming an effective tumor immune response. In addition, immune checkpoints can be used by tumors in the tumor tissue to develop immune escape. Immune checkpoint inhibitors act on immune checkpoints to enhance the immune response or attenuate immune suppression (1-3). However, an overactive immune response can lead to many immune-related adverse events (irAEs), which are being reported with increasing frequency. Skin, gastrointestinal and musculoskeletal systems, and lungs are most commonly affected by irAEs (4, 5).
Studies (5-7) have confirmed that ICI-related hepatitis is an immune-mediated hepatitis and a new type of drug-induced liver injury (DILI). The severity of hepatotoxicity is usually graded according to the common terminology criteria for adverse events (CTCAE) v. 4.03 (8), which uses total bilirubin (TBIL), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) as evaluation factors. The drug-induced liver injury network (DILIN) severity index takes into account the international normalized ratio (INR), symptoms (ascites or encephalopathy), and other organ failures (9). Some studies have shown that ICI-related hepatitis often leads to the discontinuation of therapy and can be life-threatening in the absence of specific treatment (10, 11). Corticosteroids are the most common initial treatment. However, even corticosteroids combined with immunosuppressant treatment do not effectively treat grade 3 and 4 liver injury, and deaths are commonly reported (5, 12, 13). Furthermore, long-term use of glucocorticoids may cause several adverse events (AEs) and superinfection (4, 14).
The artificial liver support system (ALSS) has been used for treating severe liver damage. Both plasma exchange (PE) and a double-plasma molecular adsorption system (DPMAS) are widely utilized (15-17). With PE, the patient's plasma is separated from blood by a plasma separator to remove toxic substances adsorbed by plasma proteins and dissolved in the plasma, and then fresh frozen plasma containing coagulation factors and albumin (ALB) is transfused back into the body, along with the blood (15). In DPMAS, large- and medium-size soluble bilirubin and inflammatory mediators are removed when the filtered plasma is passed through a specific bilirubin adsorber and macroporous resin, respectively (16, 17). Studies have reported that both DPMAS and PE are promising treatments for severe viral hepatitis and liver failure, and DPMAS combined with PE is more effective than PE or DPMAS alone (16, 17). Still, clinical trial data (18-20) on the use of ALSS in patients with ICI-related hepatitis are limited (Table 1).
| Case (n) | Diagnosis | Cancer Treatment | ALSS | Treatment Outcome | |
|---|---|---|---|---|---|
| Chen et al. (18) | 1 | Hepatocellular carcinoma | Toripalimab | PE combined with CRRT | Died of multiple organ dysfunction syndrome 71 hours after liver transplantation |
| Wu et al. (19) | 1 | Hepatocellular carcinoma with pulmonary metastasis | Sorafenib with sequential 1 course of pembrolizumab | PE | Died of liver failure and its complications |
| Tan et al. (20) | 1 | Esophageal cancer with lymph node metastasis | 2 courses of camrelizumab + capecitabine | 5 rounds of PE with sequential DPMAS | Appetite improved, and TBIL decreased. Liver function was normalized 4 weeks after discharge. |
Abbreviations: ALSS, artificial liver support system; DPMAS, double-plasma molecular absorption system; PE, plasma exchange; CRRT, continuous renal replacement therapy; TBIL, total bilirubin.
2. Objectives
This study aimed to investigate the effectiveness of PE and DPMAS for ICI-related hepatitis.
3. Methods
3.1. Patient Selection
This retrospective analysis was conducted on adult patients (≥ 18 years old) with ICI-related hepatitis treated at the Third Affiliated Hospital of Sun Yat-Sen University (SYSU), China. Diagnosis of ICI-related hepatitis was based on medical history, clinical features, and laboratory studies. The other inclusion criteria were: (1) no history of jaundice, hepatitis, or alcohol abuse; (2) no active viral infection, including viral hepatitis A, B, C, and E, Epstein-Barr virus, herpes simplex virus, cytomegalovirus, varicella-zoster virus, and human immunodeficiency virus, excluded by laboratory testing; (3) normal levels of hemoglobin A (HbA), hemoglobin B (HbA2), and fetal hemoglobin (HbF); (4) negative Coombs test, Ham's test, and Rous test; (5) normal serum ceruloplasmin and copper levels; (6) no Kayser-Fleischer ring observed upon examination by an experienced ophthalmologist; (7) normal serum α-1-antitrypsin concentration, thyroid function tests, and alpha-fetoprotein level. The exclusion criteria were contraindications to ALSS, including active hemorrhage, disseminated intravascular coagulation (DIC), heart dysfunction, unstable postmyocardial or cerebral infarction, and allergic reaction to plasma, protamine, or heparin.
3.2. Treatment
The ICI treatment was stopped. Some patients only received comprehensive medical treatments, including general supportive care, as well as energy and vitamin supplementation.
The other patients were treated with an ALSS. Plasma exchange was performed with a plasma separator (MICROPLAS MPS 07, Belk Co. Ltd., Italy). About 2,000 mL of plasma was exchanged at each session. When DPMAS + PE was performed, the plasma flowed sequentially through an ion exchange resin (BS330, Zhuhai Health Sales Biotechnology Co., Ltd, China) and a neutral macroporous adsorption resin (HA330-II, Zhuhai Health Sales Biotechnology Co., China). Approximately 5 L was filtered each session. Then, PE was performed using plasma replacement (1,000 mL). The treatments were performed every 2 to 4 days.
Complaints and AEs that occurred during treatment were monitored and recorded. These included rash, fever, jaundice, fatigue, changes in appetite, abdominal distension, hepatic encephalopathy, ascites, hemoglobinuria, and hemorrhage.
3.3. Data Analysis
Based on CTCAE v. 4.03 (8), DILIN (9), and guidelines for the diagnosis and treatment of liver failure (China, 2018) (14), the following data were extracted from medical records: TBIL, direct bilirubin (DBIL), GGT, ALP, AST, ALT, ALB, GLB, INR, procalcitonin (PCT), white blood cell (WBC) count, hemoglobulin (HGB) level, and platelet (PLT) count. The model for end-stage liver disease (MELD) score was calculated as MELD = 3.78 ln [TBIL (mg/dL)] + 11.2 ln (INR) + 9.6 ln [creatinine (mg/dL)] + 6.4 (pathogeny: Biliary or alcoholic 0, other 1) (21). All the patients were tested for antimitochondrial antibodies (AMA). The degree of DILI was divided into 4 grades, from 1 to 4 (8).
The data before and after the treatment are presented as mean ± standard deviation (SD) and were compared by a t-test in SPSS v. 22.0 (IBM Corp., Armonk, NY, USA). P-values of < 0.05 were considered significant.
4. Results
From June 2021 to January 2023, 16 patients were included in the study. The ICIs used by the patients and the period of ICI treatment when hepatitis occurred are shown in Table 2. Table 2 also presents the chief complaints, ICI-related hepatitis grade, ALSS methods, and anti-immune therapy. Only 1 patient (case 10) was AMA-positive (titer 1:640). All the other patients were AMA-negative. All the patients were diagnosed with ICI-related hepatitis. No patient fulfilled the diagnostic criteria of autoimmune hepatitis. All of them stopped ICI treatment immediately. Eight patients in group A received general support. The other 8 patients in group B received general support, plus 3 rounds of ALSS every 2 - 4 days (4 patients received PE and 4 received DPMAS + PE). The mean age of group A was 49.6 ± 10.3 years, and that of group B was 52.6 ± 7.9 years (t = 0.656, P = 0.523). No significant difference was found in the number of treatment days between the two groups (t = 0.000, P = 1.000).
| No. | Sex | Age (y) | Diagnosis | ICIs | Chief Complaint | Grade of Hepatitis | ALSS | Anti-immune Therapy |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 35 | Adenocarcinoma of the descending colon | 5-Fluorouracil for 62 days, oxaliplatin + cetuximab for 5 courses | Eye and skin icterus, dark urine, poor appetite for 1 week | 3 | no | No glucocorticoid or immunosuppressant |
| 2 | F | 37 | Invasive ductal carcinoma of the right breast | DS-8201 for 3 courses | Recurrent abdominal distension for 3 months, exacerbation for 10 days | 3 | no | No glucocorticoid or immunosuppressant |
| 3 | M | 41 | Adenocarcinoma of ascending colon | Bevacizumab + FOLFIRINOX for 2 courses | Epigastric pain, poor appetite, fatigue for 4 weeks | 3 | no | No glucocorticoid or immunosuppressant |
| 4 | F | 59 | Adenocarcinoma of right upper lung | Pemetrexed disodium + sintilimab for 8 courses | Poor appetite, dark urine, eye and skin icterus for 20 days | 3 | no | Methylprednisolone 100 mg daily for 4 days |
| 5 | M | 54 | Clear cell carcinoma of the left kidney | Tislelizumab for 3 courses | Fatigue and poor appetite for 3 days | 3 | no | Methylprednisolone 40 mg daily for 6 days |
| 6 | F | 54 | Invasive ductal carcinoma of the right breast | Pertuzumab + trastuzumab + taxane for 3 courses, sequential pyrotinib + taxane for 1 course | Fatigue, poor appetite, dark urine, eye and skin icterus for 10 days | 3 | no | No glucocorticoid or immunosuppressant |
| 7 | F | 60 | Invasive ductal carcinoma of the right breast | Tirelizumab + bevacizumab + taxane for 3 courses, sequential bevacizumab + taxane for 3 courses | Eye and skin icterus for 11 days | 4 | no | No glucocorticoid or immunosuppressant |
| 8 | F | 57 | Papillary adenocarcinoma of the right breast | Exemestane + desulumab + abemaciclib for 1 course | Fatigue and poor appetite for 22 days | 4 | no | No glucocorticoid or immunosuppressant |
| 9 | M | 42 | Nasopharyngeal carcinoma | Camrelizumab + gemcitabine + cisplatin for 1 course | Eye and skin icterus for 2 weeks | 4 | DPMAS + PE | No glucocorticoid or immunosuppressant |
| 10 | M | 47 | Nasopharyngeal carcinoma | Gemcitabine + camrelizumab + nedaplatin for 1 course, sequential camrelizumab for 2 courses | Eye and skin icterus for 6 weeks | 4 | DPMAS + PE | Methylprednisolone 12.0 mg daily for 6 days |
| 11 | F | 49 | Adenocarcinoma of ascending colon | FOLFOX for 5 courses, sequential toripalimab for 2 courses | Eye and skin icterus for 7 days | 4 | DPMAS + PE | No glucocorticoid or immunosuppressant |
| 12 | M | 66 | Mucous adenocarcinoma of the gastric fundus | FOLFOX and nivolumab for 3 courses | Fatigue, poor appetite, dark urine for 3 days | 4 | PE | Mycophenolate mofetil 1 g daily for 3 days, sequential tacrolimus 3.0 mg + methylprednisolone 30 mg daily for 6 days |
| 13 | M | 50 | Mucous adenocarcinoma of the gastric body | Tirelizumab + gimeracil + tegafur + oteracil potassium for 3 courses | Skin and eyes icterus for more than 1 month | 3 | PE | Mycophenolate mofetil 1.0 g + dexamethasone 10.0 mg daily for 6 days |
| 14 | F | 50 | Peripheral adenocarcinoma of the left lung | Pemetrexed disodium + cisplatin, sequential sintilimab for the course each | Fatigue, cough, expectoration, and fever for 3 days | 4 | PE | No glucocorticoid or immunosuppressant |
| 15 | M | 58 | Clear cell carcinoma of the right kidney | Sintilimab for 7 courses, sequential sorafenib for 2 weeks | Poor appetite for 1 month, eye and skin icterus for 15 days | 4 | DPMAS + PE | No glucocorticoid or immunosuppressant |
| 16 | M | 58 | Adenocarcinoma of the right upper lung | Erlotinib for half a year | Eyes and skin icterus, gray stool for 20 days | 4 | DPMAS + PE | No glucocorticoid or immunosuppressant |
Abbreviations: ICIs, immune checkpoint inhibitors; ALSS, artificial liver support system; DPMAS, double-plasma molecular absorption system; PE, plasma exchange.
Before the treatment, there was no significant difference in DBIL, GGT, ALP, AST, ALT, PCT, INR, MELD, ALB, GLB, WBC count, PLT count, or HGB between the two groups (P > 0.05 in all cases). However, the TBIL of group B was significantly higher than that of group A (P = 0.029).
After treatment, TBIL and DBIL were significantly decreased in group B (both P < 0.05; Table 3). In addition, group B had a significantly lower GGT (P = 0.028) and higher INR (P = 0.004) than group A (Table 4). The ALP level of group B was also lower, but the difference was not significant (P = 0.068; Table 4).
| Items | Group A | Group B | ||
|---|---|---|---|---|
| t | P | t | P | |
| TBIL (umol/L) | -0.239 | 0.815 | 3.038 | 0.013 |
| DBIL (umol/L) | -0.155 | 0.879 | 3.062 | 0.010 |
| GGT (U/L) | -0.062 | 0.952 | 1.753 | 0.119 |
| ALP (U/L) | -0.226 | 0.852 | 1.664 | 0.136 |
| AST (U/L) | 1.200 | 0.250 | 1.014 | 0.328 |
| ALT (U/L) | 0.774 | 0.452 | 0.860 | 0.404 |
| PCT (ng/mL) | 0.548 | 0.598 | 0.809 | 0.432 |
| WBC (E9/L) | 0.161 | 0.875 | -0.131 | 0.897 |
| INR | 1.374 | 0.209 | -0.991 | 0.338 |
| MELD | 0.675 | 0.510 | 0.472 | 0.645 |
| ALB (g/L) | -0.293 | 0.776 | 0.554 | 0.589 |
| GLB (g/L) | 0.167 | 0.870 | 1.194 | 0.252 |
| PLT (E9/L) | -1.508 | 0.153 | 0.329 | 0.747 |
| HGB (g/L) | 0.774 | 0.454 | 1.524 | 0.150 |
Abbreviations: TBIL, total bilirubin; DBIL, direct bilirubin; GGT, glutamine transpeptidase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PCT, procalcitonin; WBC, white blood cell; INR, international normalized ratio; MELD, model for end-stage liver disease; ALB, albumin; PLT, platelet; HGB, hemoglobulin.
| Items | Before Treatment | After Treatment | ||||||
|---|---|---|---|---|---|---|---|---|
| Group A | Group B | t | P | Group A | Group B | t | P | |
| TBIL (umol/L) | 115.51 ± 124.20 | 279.6 ± 145.4 | 2.427 | 0.029 | 131.6 ± 144.4 | 108.4 ± 65.2 | -0.414 | 0.685 |
| DBIL (umol/L) | 77.13 ± 90.21 | 163.8 ± 74.0 | 2.102 | 0.054 | 84.5 ± 98.8 | 69.4 ± 45.4 | -0.380 | 0.710 |
| GGT (U/L) | 550.3 ± 355.9 | 529.8 ± 540.9 | -0.005 | 0.933 | 562.8 ± 419.4 | 186.8 ± 117.4 | -2.442 | 0.028 |
| ALP (U/L) | 611.9 ± 574.1 | 545.9 ± 519.6 | -0.234 | 0.819 | 682.9 ± 631.2 | 231.1 ± 128.3 | -1.969 | 0.068 |
| AST (U/L) | 261.0 ± 149.0 | 413.1 ± 749.8 | 0.563 | 0.582 | 179.6 ± 120.7 | 133.3 ± 128.4 | -0.744 | 0.469 |
| ALT (U/L) | 198.9 ± 253.3 | 406.1 ± 797.7 | 0.700 | 0.493 | 124.0 ± 103.3 | 153.5 ± 236.2 | 0.324 | 0.751 |
| PCT (ng/mL) | 1.85 ± 4.35 | 0.40 ± 0.37 | -0.941 | 0.364 | 0.42 ± 0.46 | 0.28 ± 0.25 | -0.705 | 0.499 |
| WBC (E9/L) | 7.73 ± 9.14 | 9.18 ± 6.88 | 0.359 | 0.725 | 7.08 ± 4.20 | 10.74 ± 8.13 | 0.998 | 0.338 |
| INR | 1.14 ± 0.40 | 1.17 ± 0.34 | 0.174 | 0.864 | 0.94 ± 0.08 | 1.30 ± 0.24 | 3.92 | 0.004 |
| MELD | 8.57 ± 10.88 | 11.40 ± 3.44 | 0.72 | 0.494 | 5.47 ± 7.09 | 8.81 ± 5.56 | 1.050 | 0.312 |
| ALB (g/L) | 35.0 ± 6.3 | 34.8 ± 3.7 | -0.077 | 0.939 | 34.2 ± 2.5 | 33.6 ± 4.5 | -0.331 | 0.746 |
| GLB (g/L) | 29.0 ± 6.2 | 25.5 ± 5.2 | -1.029 | 0.239 | 28.4 ± 7.4 | 22.6 ± 4.5 | -1.876 | 0.083 |
| PLT (E9/L) | 200 ± 69 | 198 ± 70 | -0.043 | 0.966 | 265 ± 91 | 189 ± 37 | -2.158 | 0.052 |
| HGB (g/L) | 113 ± 17 | 102 ± 10 | -1.630 | 0.125 | 105 ± 21 | 94 ± 11 | -1.356 | 0.200 |
Abbreviations: TBIL, total bilirubin; DBIL, direct bilirubin; GGT, glutamine transpeptidase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PCT, procalcitonin; WBC, white blood cell; INR, international normalized ratio; MELD, model for end-stage liver disease; ALB, albumin; PLT, platelet; HGB, hemoglobulin.
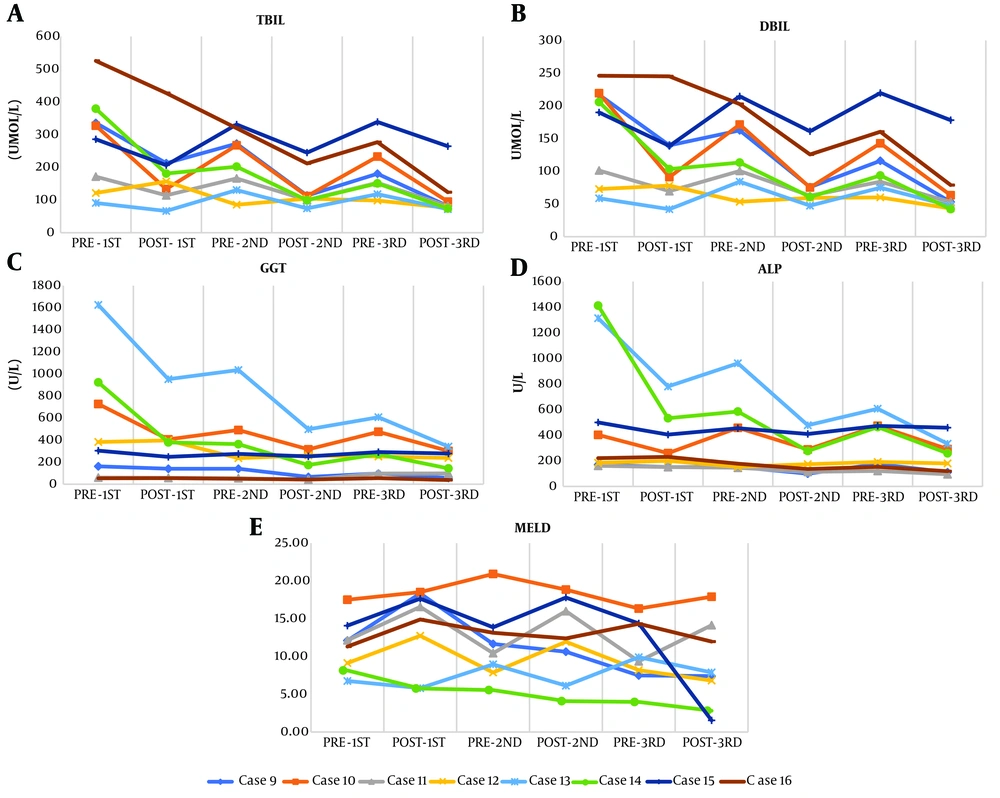
The changes in TBIL, DBIL, GGT, ALP, and the MELD score during each session of ALSS are shown in Figure 1. All MELD scores were below 21. No significant differences were found in AST, ALT, PCT, WBC count, INR, MELD score, ALB, GLB, PLT count, or HGB between the two groups (P > 0.05 in all cases; Table 4).
The chief complaints were improved. No allergic reaction or severe adverse effect was observed, including rash, fever, ascites, hemoglobinuria, hemorrhage, or hepatic encephalopathy.
5. Discussion
Based on the molecular mechanism, ICIs are commonly divided into three categories: Anti-cytotoxic T-lymphocyte-associated molecule-4 (CTL-4) antibody, anti-programmed cell death receptor-1 (PD-1) antibody, and anti-programmed cell death ligand-1 (PDL-1) antibody. Anti-programmed cell death receptor-1 is expressed on the surface of T cells, while its ligand is expressed on the surfaces of tumor cells and myeloid suppressor cells. Immune checkpoint inhibitors inhibit the inflammatory activity of T lymphocytes by binding to the PDL1/PDL2 ligand, thereby improving the host's immune response against tumor cells (4, 10). However, an excessive immune response can cause liver damage. Reports of ICI-related hepatitis are increasing, and related drugs include camrelizumab, toripalimab, and nivolumab (18-20).
The results of our study showed that ICI-related hepatitis can occur with different ICIs. Peeraphatdit et al. (5) reported that the incidence of ICI-related hepatitis ranged from 0.7% to 16%, and the frequency was affected by treatment protocol, dosage, and patient characteristics. According to De Martin et al. (7), patients usually presented with mixed hepatocellular and cholestatic liver injury, with inflammation and necrosis of liver cells occurring at the peak of the disease.
Notably, our results revealed that serum TBIL and DBIL were significantly decreased after ALSS treatment in group B (both P < 0.05). The ALP level was also lower, but the difference was not significant (P = 0.068). In addition, group B had a significantly lower GGT (P = 0.028) and higher INR (P = 0.004) than group A. As shown in Figure 1, the changes in TBIL, DBIL, GGT, ALP, and the MELD score during each session of ALSS support the fact that both PE and DPMAS + PE can retard the progression of the condition and inflammatory response. These results are consistent with those reported by Tan et al. (20). Larsen (22) also found that PE had a positive effect on ICI-related liver injury by decreasing bilirubin, creatinine, and inflammatory factors in the plasma. Reeves and Winters (23) also reported that PE increased suppressor T function but decreased B cells. In that study, after treatment, the ratio of helper T cell type 1/2 (Th1/Th2) was increased. Chen et al. (24) and Li et al. (25) demonstrated a decline in the serum level of interleukin (IL)-6, which can induce adhesion and aggregation of inflammatory cells to promote inflammation. Xia et al. (26) reported that the levels of WBC, PCT, IL-6, IL-10, and C-reactive protein were effectively decreased after ALSS (all P < 0.05). The proportions of patients with these abnormal inflammation-related indicators were also significantly reduced (P < 0.05). Taken together, our results and those of the aforementioned studies indicate that PE and DPMAS + PE can reduce hepatotoxicity and promote a beneficial environment for hepatocyte regeneration and repair.
As depicted in Tables 3 and 4 and Figure 1, all MELD scores were less than 21. Our results support that immediate termination of the ICI and timely treatment can improve the prognosis of ICI-related hepatitis. Furthermore, the results support monitoring liver function during ICI therapy.
Hepatotoxicity is a significant irAE. In most reported cases of ICI-related hepatitis, the ICI was stopped, and immunosuppressive treatment started. Notably, in our study, 1 patient was positive for AMA (titer 1:640). The finding suggests the complexity of ICI-related hepatitis and that it can be caused by a variety of pathogenic mechanisms. Based on our results, we encourage the use of ALSS in the treatment of ICI-related hepatitis.
Because ICIs have become available for the treatment of cancers in China only recently, the number of cases of ICI-related hepatitis is limited, and the number of patients treated with ALSS is very small. Our study was a retrospective analysis of patients treated in our hospital. Thus, there was no sample size or power calculation. We plan a future study in which sample size calculation for statistical analysis will be performed with more cases. We also plan to include data such as liver histology and immunological items.
5.1. Conclusions
Many ICIs can cause ICI-related hepatitis, and the condition can present at different times during treatment. Both PE and DPMAS + PE effectively improve ICI-related hepatitis in the short term and may be more useful in patients with hyperbilirubinemia. Liver function should be monitored continuously during ICI therapy. Immediate ICI termination and timely treatment can improve the prognosis of ICI-related hepatitis.