1. Context
Hepatic encephalopathy (HE) originates from acute liver failure or hepatic liver injury leading to severe brain function impairment and is defined as a neurological disorder secondary to liver dysfunction and/or portosystemic shunt. Psychiatric syndrome, which is characterized by extensive neuropsychiatric changes ranging from subclinical changes to coma, is a continuous neurocognitive dysfunction (1). In 2014, the American Association for the Study of Liver Disease divided HE into type A (acute type), type B (bypass type), and type C (cirrhosis type) according to etiology. According to clinical severity, patients are divided into covert HE (CHE) and overt HE, with the former including minimal HE (MHE) and West Haven grade I HE and the latter including West Haven grades II-IV HE (2). Different types of HE may affect the results of diagnosis and treatment. The incidence of HE is high, and about 80% of patients with the CHE type will experience MHE (1). Accordingly, the diagnosis of MHE is critical. As the condition of patients with HE worsens, their quality of life decreases; therefore, early diagnosis helps intervene in the disease (1).
There are various diagnostic methods for MHE, such as intelligence tests, neurophysiological examination, serum marker detection, and imaging examination, each with its advantages and disadvantages. Among these, magnetic resonance imaging (MRI) is non-invasive enough to evaluate brain parenchymal damage in patients with early HE from multiple aspects, thereby providing a new approach to understanding the pathophysiological manifestations of HE and improving the accuracy of HE diagnosis (3). With the application of various MRI techniques and analysis methods in MHE, increasing studies have found different degrees of change in the structure, metabolism, and function of multiple brain regions in patients with MHE. For example, Chen et al. (4) measured the spontaneous activity of the brain by detecting the amplitude of low-frequency fluctuations (ALFF) and analyzed the results of diagnosing MHE using psychometric HE scores (PHES). The results showed that the accuracy of ALFF values in diagnosing MHE was 80.6%, the sensitivity was 81.3%, and the specificity was 80.0%. In addition, MRI can describe the centrality of different nodes in the brain network and detect changes in the topological functional network of connections between different nodes and the nodes of the whole-brain functional network (5); this can be used to quantify each node in the brain network and determine the abnormal brain area of the whole-brain functional connection. At present, MRI is widely used to reveal the mechanism of other neuropsychological diseases, such as Alzheimer’s disease, type 2 diabetes cognitive impairment, and depression (6-8), and the method is gradually being applied to the study of cognitive function in patients with HE. Chen et al. (9) used this method to find abnormalities in the intrinsic functional connectivity of multiple brain regions in patients with HE. A meta-analysis of the correlation between cognitive function and imaging in HE as early as possible can effectively determine the effect of imaging in the diagnosis of cognitive function in patients with HE, enabling the prompt preventive treatment of cognitive impairment in such patients.
2. Methods
2.1. Search Strategy
We systematically searched 2 Chinese and 1 English databases, namely China National Knowledge Infrastructure, Wanfang, and PubMed. The retrieval time was from the establishment of the databases to March 20, 2023. The keywords used included ‘Hepatic encephalopathy’, ‘Minimal hepatic encephalopathy’, ‘Covert hepatic encephalopathy’, ‘Cognitive impairment’, ‘Cognition disorders’, ‘Magnetic resonance imaging’, ‘MRI’ and ‘Imaging’; the keywords in Chinese included the equivalents of ‘Cognitive function’, ‘Cognitive impairment’, ‘Hepatic encephalopathy’, ‘Mild hepatic encephalopathy’, ‘Imaging’, ‘Magnetic resonance imaging’ and ‘MRI’. We used ‘AND’ and ‘OR’ to connect these words as follows: (‘Cognitive impairment’ OR ‘Cognition disorders’ OR ‘Cognitive function’) AND (‘Hepatic encephalopathy’ OR ‘Minimal hepatic encephalopathy’ OR ‘Covert hepatic encephalopathy’ OR ‘Mild hepatic encephalopathy’) AND (‘Magnetic resonance imaging’ OR ‘MRI’ OR ‘Imaging’). The literature was retrieved using the subject retrieval method, and additional target literature was retrieved according to the references of the previously retrieved literature.
2.2. Inclusion and Exclusion Criteria
Inclusion criteria: (1) Chinese and English studies published in peer-reviewed journals; (2) subjects with mild hepatic encephalopathy, subclinical hepatic encephalopathy, hepatic encephalopathy, or dominant hepatic encephalopathy; (3) the diagnostic method being imaging examination; (4) observation indices being used to determine cognitive function in HE, such as digit connection test reaction times, digit symbol test (DST) scores and psychometric HE scores (PHES).
Exclusion criteria: (1) Studies in which the diagnosis was not made by imaging; (2) observation indices that did not include the study of cognitive function in patients with HE; (3) insufficient information on outcomes and an inability to perform data analysis; (4) repeated reports of literature research; (5) incomplete research articles; (6) lack of data reporting on research results; (7) conference articles, case reports, and systematic reviews.
2.3. Literature Screening and Data Extraction
Two researchers performed literature screening separately based on the inclusion and exclusion criteria by reading the titles and abstracts of the studies for initial screening and then by reading the full text of the studies that might have met the inclusion criteria. When the two researchers disagreed, the opinion of a third researcher was sought, and a discussion took place to reach a consensus. After the literature screening was completed, two researchers carried out data extraction according to an established standard data extraction form. The extracted information included literature information, research type, time of publication, demographic characteristics of the subjects, imaging diagnostic methods, cognitive function indicators, and correlation coefficients between imaging results and cognitive function indicators.
2.4. Quality Assessment
The risk of bias in the included literature was assessed using the revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) (10). The QUADAS-2 tool evaluates 14 biased items in 4 aspects: Case selection, trial to be evaluated, gold standard, case flow, and progression. Each article was assessed for bias according to 3 criteria (‘yes’, ‘no’, and ‘unclear’), which evaluated whether the article complied with the 14 items. Grade-A literature with ≥10 items met the ‘yes’ criterion, with a very low risk of related bias. Grade-B literature met only part of the evaluation criteria, and there was a slight possibility of bias. Assessed by two researchers, in the case of a disagreement, consensus was reached after consultation or judgment with an additional expert.
2.5. Statistical Methods
The data were converted as follows and then meta-analyzed using RevMan v. 5.3 software. The conversion formula was as follows (11):
(Z is the sum Fisher’ sZ value)
For data where the outcome variable was the correlation coefficient (R-value), Fisher’s Z was converted from the above formula, and Fisher’s Z and the standard error (SE) were input to RevMan v. 5.3 software to obtain the summary Fisher’s Z value using the inverse variance method (12). Finally, the summary R-value was converted from Formula 4. The presence of heterogeneity in the studies was determined by chi-squared testing, and a fixed-effects model was chosen if I2 < 50% or P > 0.1 could be considered homogeneous across multiple homogeneous studies. A random-effects model was chosen if I2 > 50% or P ≤ 0.1. Finally, the summary R-value was used to evaluate the correlation between imaging diagnosis and cognitive function in patients with HE.
In general, the correlative strength of the variables was determined by the range of the absolute values of the correlation coefficient R: 0.8 - 1.0 indicated a very strong correlation, 0.6 - 0.8 indicated a strong correlation, 0.4 - 0.6 indicated a moderate correlation, 0.2 - 0.4 indicated a weak correlation and 0.0 - 0.2 indicated a very weak correlation or no correlation.
3. Results
3.1. Basic Study Characteristics and the Results of the Literature Quality Assessment
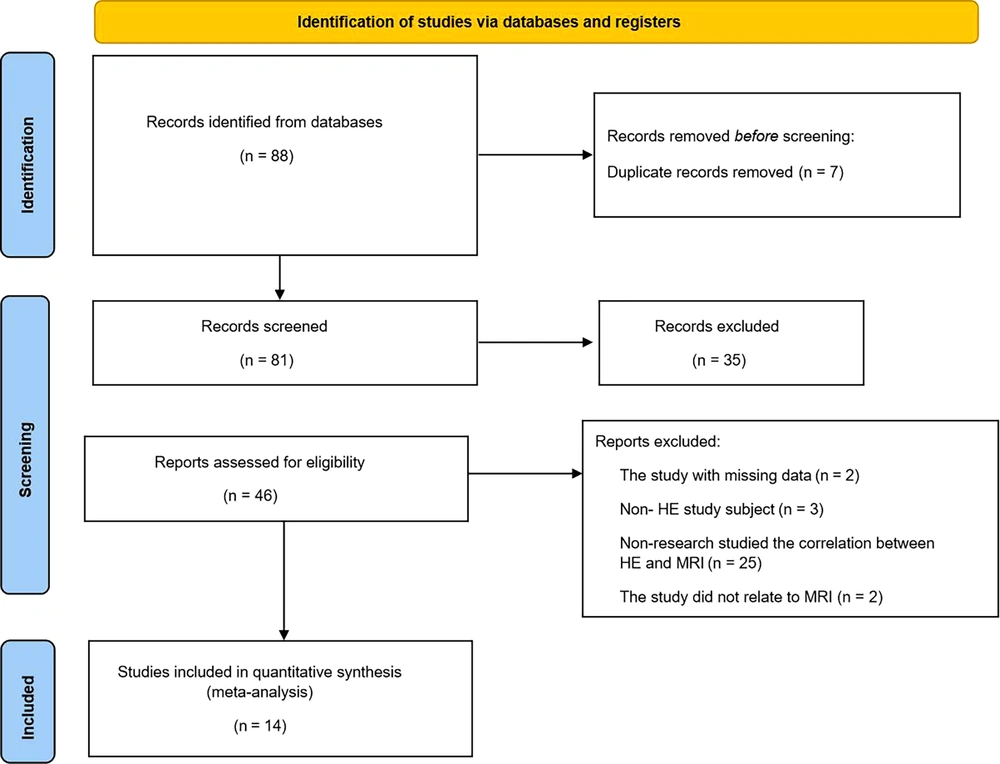
After a systematic search of Chinese and English databases, 88 articles passed the preliminary screening. After the titles and abstracts were examined, 35 articles were found to be irrelevant to this study and were excluded. Forty-six articles were subjected to full-text reviews, resulting in 14 studies meeting the inclusion criteria for this paper. Two articles were excluded due to missing data, 3 articles had non-HE subjects, 25 articles did not study the correlation between HE and MRI, and 2 studies were not related to MRI. The literature screening process is shown in Figure 1. The 14 studies involved 622 subjects; 10 studies were case-control studies, and the rest were cross-sectional studies. The subjects were patients with mild HE, patients with simple liver cirrhosis, and healthy people, and they were between 40 and 60 years old. The proportion of men in each study was greater than that of women. The basic characteristics of the included studies are listed in Table 1 (13-26). In addition, the included studies were high-quality literature. According to the results of QUADAS-2, 12 studies had ≥10 items that met “yes", which were Grade-A studies, and only 2 were Grade-B studies. The specific quality evaluation is presented in Table 2.
| Study Number | Research Design | Age ( | Male/Female (Case) | Objects | Imaging Diagnosis | Cognitive Function Diagnosis | Correlation Coefficient | R and P-Values |
|---|---|---|---|---|---|---|---|---|
| Zhou, 2021 (13) | Case-control | 49.44 ± 9.57 | 31/10 | Healthy and mild hepatic encephalopathy | 3.0T MRI | PHES | Spearman | PHES and frontal lobe area: r = 0.59, P = 0.006; PHES and gray matter: r = 0.646, P = 0.002 |
| Wang, 2019 (14) | Case-control | 50.16 ± 3.80 | 39/21 | Patients with simple liver cirrhosis, mild hepatic encephalopathy, and healthy subjects | 3.0T MRI | NCT-A, DST | Pearson | NCT-A and occipital lobe: r = 0.319, p = 0.045; DST and occipital lobe: r = -0.325 p = 0.04 |
| Yang et al., 2023 (15) | Cross-section study | 47.00 ± 9.30 | 19/9 | Patients with mild hepatic encephalopathy | fMRI | NCT-A, DST, MoCA scale | Person | - |
| Shi, 2015 (16) | Case-control | 45.23 ± 9.30 | 68/28 | Patients with simple liver cirrhosis, mild hepatic encephalopathy, and healthy subjects | fMRI | NCT-A, DST | Spearman | NCT-A and frontal lobe area: r = -0.45, P = 0.01; DST and frontal lobe area: r = 0.48, P = 0.005 |
| Zheng et al., 2014 (17) | Case-control | 51.86 ± 9.74 | 18/12 | Patients with mild hepatic encephalopathy and healthy subjects. | 3.0T MRI | NCT-A, DST | Pearson | NCT-A and frontal lobe area: r = 0.188, P = 0.319; NCT-A and occipital lobe: r = 0.202, P = 0.285; NCT-A and cerebral pallidus: r = -0.266, P = 0.155; DST and frontal lobe area: r = 0.435, P = 0.016;DST and occipital lobe: r = -0.489 P = 0.006;DST and cerebral pallidus: r = 0.678, P<0.001 |
| Shih, 2018 (18) | Case-control | 47.42 ± 1.74 | 43/12 | Patients with simple liver cirrhosis, mild hepatic encephalopathy, and healthy subjects | MRI | NCT-A, DCT | Pearson | NCT-A and occipital lobe: r = 0.33, P = 0.029; NCT-A and cerebral pallidus: r = -0.213, P = 0.446; DST and occipital lobe: r = 0.112, P = 0.692; DST and cerebral pallidus: r = 0.339, P = 0.216 |
| Ji et al., 2020 (19) | Case-control | 45.20 ± 7.54 | 33/19 | Patients with simple liver cirrhosis, mild hepatic encephalopathy, and healthy subjects | fMRI | NCT-A, DST | Pearson | NCT-A and frontal lobe area: r = 0.85, P < 0.001; DST and frontal lobe area: r = 0.78, P < 0.001; DST and occipital lobe: r = 0.78, P < 0.001 |
| Zhang et al., 2018 (20) | Case-control | 56.55 ± 8.51 | 34/6 | Patients with hepatic encephalopathy and healthy people | 3.0T MRI | NCT-A, DST | Pearson | NCT-A and frontal lobe area: r = 0.115, P = 0.623; NCT-A and occipital lobe: r = -0.242, P = 0.014; DST and frontal lobe area: r = 0.521, P < 0.001; NCT-A and occipital lobe: r = 0.422, P < 0.001 |
| Lv et al., 2016 (21) | Cross-section study | 44.6 ± 10.01 | 26/5 | Patients with mild hepatic encephalopathy | fMRI | PHES | Spearman | PHES and gray matter: r = 0.404, P = 0.024 |
| Cai et al., 2022 (22) | Case-control | 51.66 ± 9.31 | 42/9 | Patients with simple liver cirrhosis and mild hepatic encephalopathy | 3.0T MRI | PHES | Spearman | PHES and gray matter: r = 0.474, P = 0.047 |
| Chen et al., 2017 (23) | Cross-section study | 52.70 ± 9.90 | 16/2 | Patients with mild hepatic encephalopathy | 3.0T MRI | PHES | Spearman | PHES and gray matter: r = 0.474, P = 0.047 |
| Chen et al., 2018 (24) | Case-control | 50.56 ± 8.88 | 30/5 | Patients with cirrhosis with prior overt hepatic encephalopathy and without prior overt hepatic encephalopathy | 3.0T MRI | PHES | Pearson | PHES and gray matter: r = 0.546, P = 0.016 |
| Zhan et al., 2019 (25) | Cross-section study | 50.20 ± 9.40 | 17/2 | Minimal hepatic encephalopathy | fMRI | PHES | Spearman | PHES and frontal lobe area: r = 0.556, P = 0.044; PHES and gray matter: r = 0.477, P = 0.039 |
| Ahluwalia et al., 2016 (26) | Case-control | Missing | 66 | Patients with hepatic encephalopathy | fMRI | PHES | Pearson | DST and occipital lobe: r = 0.57, P = 0.009 |
Characteristics of the Included Studies
| Study Number | Was the Selected Patient Sample Consecutive or Random? | Was a Case-Control Design Avoided? | Did the Study Avoid Inappropriate Exclusions? | Whether You Are Concerned About a Mismatch Between Included Patients and Settings and Review Questions | Are Indicator Test Results Interpreted Without Knowledge of Reference Standard Results? | If Thresholds Are Used, Are They Pre-specified? | Whether You Are Concerned About the Index Test, its Behavior, or Its Interpretation Being Different from the Retrospective Questions | Is the Reference Standard Likely to Correctly Classify the Target Condition? | Were the Reference Standard Results Interpreted Without Knowledge of the Index Test Results? | Are You Worried That the Target Conditions Defined by the Reference Standard Do Not Match the Problem? | Is There an Appropriate Interval Between the Index Test and the Reference Standard? | Are All Patients Receiving the Same Reference Standard? | Were All Patients Included in the Analysis? | Does Patient Selection Lead to Bias? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zou, 2021 (13) | Do not know | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Do not know | Yes | Yes | Yes |
| Wang, 2019 (14) | No | No | Yes | Yes | Yes | Do not know | Yes | Yes | Yes | Yes | Yes | Do not know | Yes | Yes |
| Yang et al., 2023 (15) | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Do not know | No | No | Yes |
| Shi, 2015 (16) | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Do not know | Do not know | Yes | Yes |
| Zheng et al., 2014 (17) | Do not know | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Do not know | Yes | Yes | Yes |
| Shi YN,2018 (18) | Do not know | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Do not know | No | Yes | Yes |
| Ji et al., 2020 (19) | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Do not know | Do not know | No | Do not know |
| Zhang et al., 2018 (20) | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Do not know | Do not know | Yes | Yes |
| Lv et al., 2016 (21) | Do not know | Yes | Yes | Yes | Yes | Yes | No | Do not know | Yes | Yes | Do not know | Do not know | No | No |
| Cai et al., 2022 (22) | Do not know | No | Yes | Yes | Yes | Yes | Yes | Yyes | Yes | Yes | Do not know | Do not know | Des | Yes |
| Chen et al., 2017 (23) | Do not know | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Do not know | Do not know | Yes | Yes |
| Chen et al., 2018 (24) | Do not know | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Do not know | Do not know | No | Yes |
| Zhan et al., 2019 (25) | Do not know | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Do not know | Do not know | No | Yes |
| Ahluwalia et al., 2016 (26) | Do not know | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Do not know | Yes | No | Yes |
Quality Assessment of Included Studies
3.2. Correlation Between Cognitive Function and Brain Frontal Lobe Imaging in Patients with Hepatic Encephalopathy
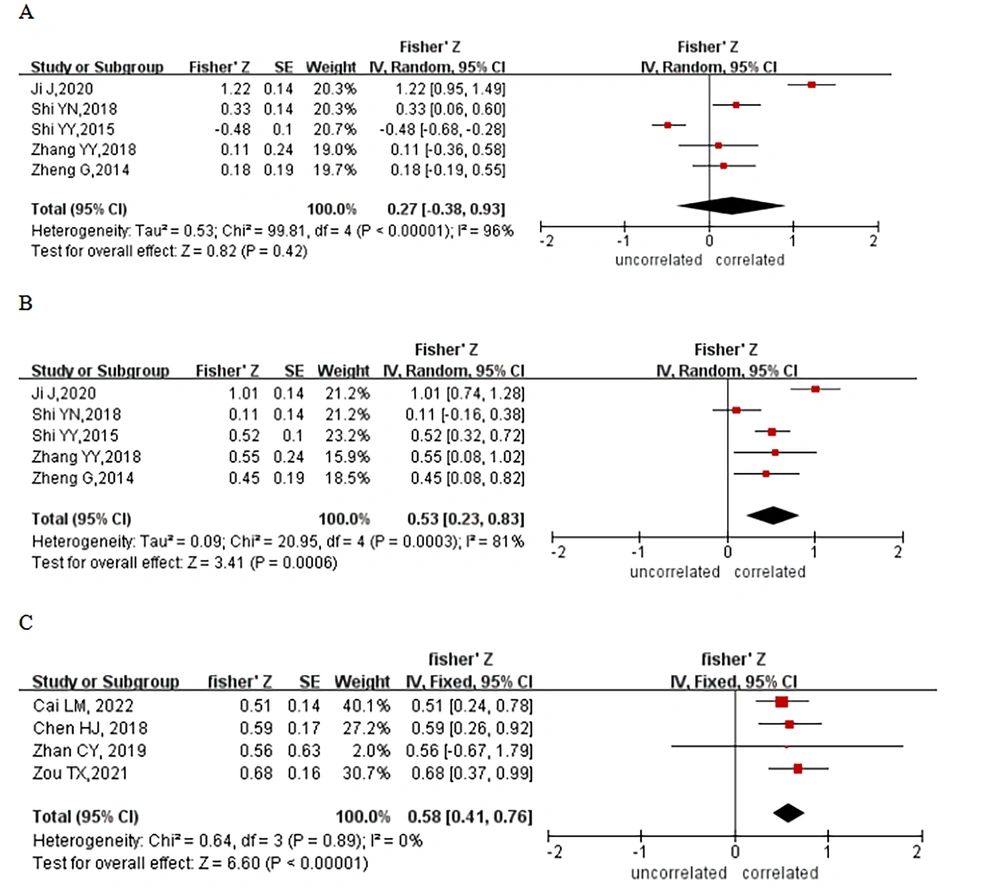
Nine studies reported correlations between cognitive function and frontal lobe imaging findings in patients with HE. Five of them used shortened number connection test-A (NCT-A) response times to assess cognitive function in patients with HE. The results of a heterogeneity analysis (I2 = 96.0%, P < 0.00001) showed some heterogeneity in the included studies, and a meta-analysis was performed using a random-effects model; however, there was no significant correlation between NCT-A response times and frontal lobe imaging findings (Z = 0.27; 95% confidence interval [CI]: - 0.38 - 0.93, Figure 2A). Five studies also used DST (DST-RRB) scores to study the correlation between cognitive function and imaging findings in patients with HE, and there was some heterogeneity among the 5 studies (I2 = 81.0%, P = 0.0003). An analysis using a random-effects model showed a positive correlation between DST scores and radiographic findings (Z = 0.53; 95% CI: 0.23 - 0.83, Figure 2B), with a correlation coefficient of 0.49. In addition, 4 studies used PHES to examine the association between cognitive function and frontal lobe imaging in HE, which was analyzed using a fixed-effects model due to heterogeneity that was not statistically significant (I2 = 0.0%, P = 0.89). The results demonstrated a correlation between PHES and the results of brain frontal imaging (Z = 0.58; 95% CI: 0.41 - 0.76, Figure 2C), with a correlation coefficient of 0.52.
3.3. Correlation Between Cognitive Function and Occipital Lobe Imaging in Patients with Hepatic Encephalopathy
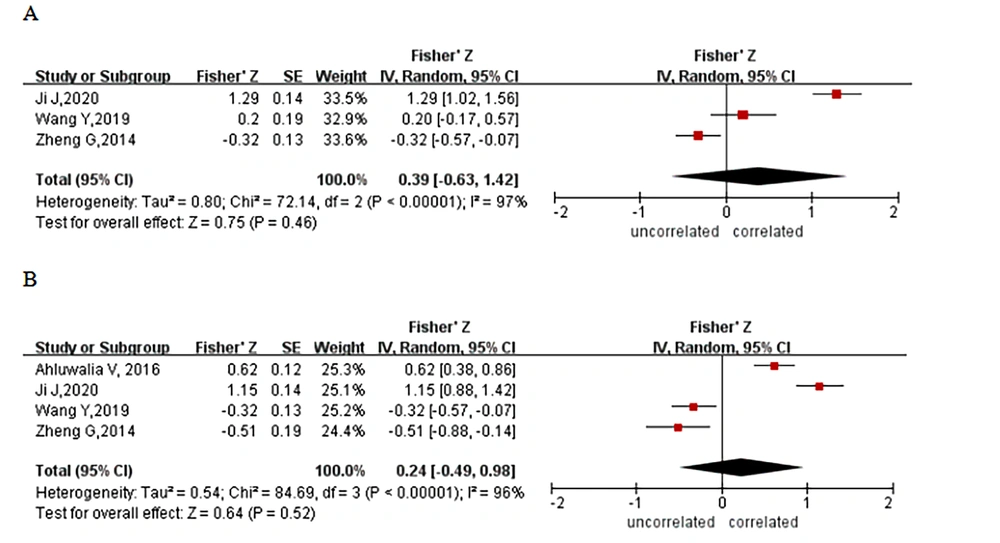
Four studies reported a correlation between cognitive function and occipital lobe imaging findings in patients with HE. Three studies analyzed the correlation between NCT-A reaction time and brain imaging results of the occipital lobe. Due to the heterogeneity of these 3 studies (I2 = 97.0%, P < 0.00001), a random-effects model analysis was performed, and the results revealed that the NCT-A reaction time was not related to the imaging results of the occipital lobe of the brain (Z = 0.39; 95% CI: - 0.63 ~ 1.42, Figure 3A). Four studies analyzed the correlation between DST scores and brain imaging results of the occipital lobe. Because of heterogeneity (I2 = 96.0%, P < 0.00001), the random-effects model was employed, and the results of the meta-analysis indicated that the DST scores and brain imaging results had no significant correlation (Z = 0.24; 95% CI: - 0.49 ~ 0.98, Figure 3B).
3.4. Correlation Between Cognitive Function and Cerebral Pallidus Imaging in Patients with Hepatic Encephalopathy
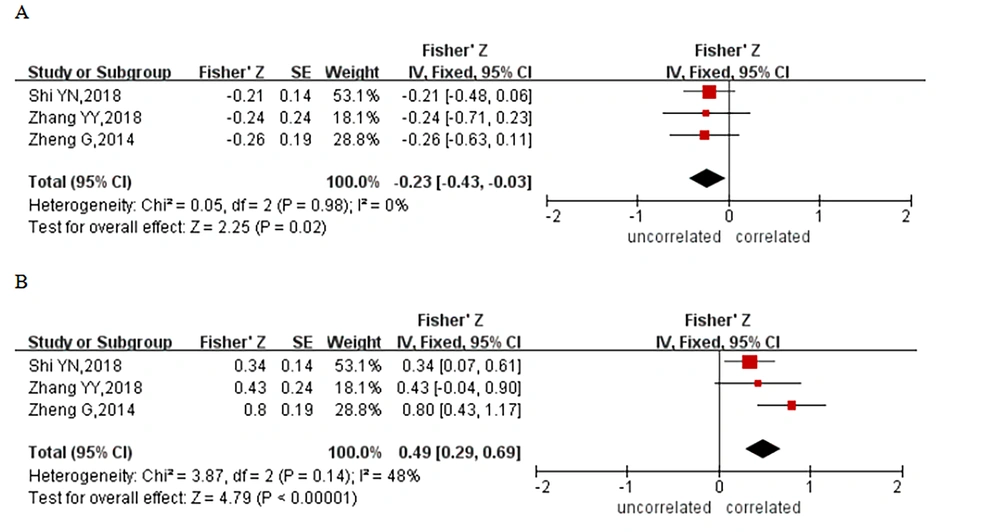
Three studies reported a correlation between cognitive function and the results of imaging results of globus pallidus in patients with HE. Three studies analyzed the association of NCT-A response time and DST score with the findings of cerebral globus pallidus imaging, and these 3 studies had heterogeneity that was not statistically significant (NCT-A: I2 = 0.0%, P = 0.98; DST: I2 = 48.0%, P = 0.14); thus, fixed-effects models were utilized. The results of a meta-analysis revealed a negative correlation between NCT-A response time and pallidography (Z = -0.23; 95% CI: - 0.43 - 0.03, Figure 4A), with a correlation coefficient of - 0.23 and a positive correlation between DST score and pallidography (Z = 0.49; 95% CI: 0.29 - 0.69, Figure 4B), with a correlation coefficient of 0.45.
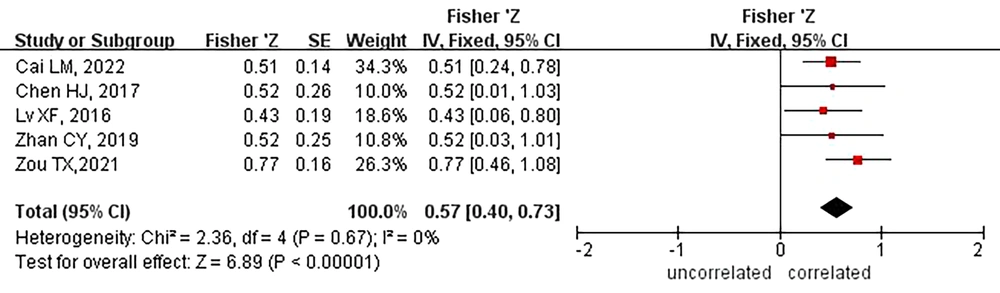
3.5. Correlation Between Cognitive Function and Grey Matter Imaging in Patients with Hepatic Encephalopathy
Five studies reported a correlation between cognitive function and grey matter imaging findings in patients with HE. The results of heterogeneity evaluation (I2 = 0.0%, P = 0.67) suggested that heterogeneity between the included studies was not significant, so the fixed-effects model was employed for systematic review.
The results of a meta-analysis indicated a positive correlation between the PHES and the mean peak of grey matter in the brain (Z = 0.57; 95% CI: 0.46 - 0.73, Figure 5), with a correlation coefficient of 0.52.
4. Discussion
A total of 14 studies that used correlation coefficients to explore the correlation between the imaging results of brain regions and cognitive function in patients with HE were included, involving a total of 622 subjects. Our results demonstrated that both DST scores and PHES were moderately positively correlated with the imaging signal values of the frontal lobe area in patients with HE; the comprehensive correlation coefficients were 0.49 and 0.52, respectively, indicating that in the frontal lobe of patients with HE, the stronger the affective signal in the region, the better the cognitive function of the patient. However, the imaging signal value of the occipital lobe in patients with HE did not correlate with the cognitive function evaluated by NCT-A reaction time and DST score.
In the study of imaging changes in the globus pallidus in patients with HE, the NCT-A reaction time and the DST score were negatively and positively correlated, respectively, with the impact signal value of the globus pallidus, with comprehensive correlation coefficients of - 0.23 and 0.45, respectively. This means that when the cognitive function of patients with HE is normal, there will be high signal imaging results in the globus pallidus, although the correlation between NCT-A reaction time and imaging results is weak. The PHES of patients with HE were also moderately and positively correlated with the mean peak of grey matter, with a correlation coefficient of 0.52. Because the signals in grey matter, the frontal lobe area, and the globus pallidus are related to cognitive function, the incidence of HE can be clinically diagnosed by observing the strength of the imaging signals in these three areas. In the future, researchers should focus on the boundary values of brain imaging signals at the time of HE.
Among the 3 evaluation methods of cognitive function, the correlation coefficient between PHES and brain imaging results is the largest, and PHES is considered to be the most accurate, which may be due to the combination of DST score, NCT-A reaction time, and other evaluation results. The PHES represents the cognitive function of patients with HE. In future studies, PHES should be used to evaluate cognitive function in patients with HE to further explore the correlation between cognitive function and brain imaging in such patients.
Shi et al. (27) studied the imaging results of patients with HE, and their findings were consistent with the results of the present study. There were significant differences in the imaging results of the frontal lobe, parietal lobe, temporal lobe, and grey matter in patients with different degrees of HE (27). In particular, changes in frontal lobe imaging can reveal, to a certain extent, the changes in cognitive function in patients with HE. The cognitive function of patients with HE is weakened, and the functional MRI low-frequency amplitude signals of the parapontine area and left supplementary motor area will also be weakened. Our study also found that cognitive function was associated with changes in globus pallidus imaging in patients with HE; this finding is similar to the results of another study on HE in patients with cirrhosis in which the globus pallidus showed stronger connectivity signals in a normal group with stronger cognitive function compared with people with cognitive impairment (28). In contrast to our research results, Li et al. (29) believed that the imaging results of the occipital lobe were related to patients with different degrees of HE.
In general, the cognitive function of HE can be determined to a certain extent by imaging. Hepatic encephalopathy will have a serious impact on people’s daily lives and work, but it is a reversible disease with a good prognosis with early diagnosis and treatment. However, due to its complex pathophysiological mechanism, there is no gold standard for its diagnosis and treatment. Currently, we can confirm the relationship between imaging results and patients. There is a correlation between HE and brain imaging, and more brain imaging sites should be identified to determine the cognitive function of patients with HE.
This study had certain limitations. First, there may have been heterogeneity among the included studies due to differences in the demographic characteristics of the subjects, imaging diagnostic instruments, and the inclusion of only English and Chinese articles. Second, most studies were case-control studies and included not only patients with HE but also those with cirrhosis without HE and healthy volunteers, and this may have weakened the correlation between cognitive function and brain imaging, and the results did not become statistically significant. Third, most studies did not mention the time interval between the measurement of cognitive function and imaging. It is uncertain whether there is a problem with time intervals being too long, which weakens the correlation between the two. Finally, each study used a variety of research indicators, which were not unified across studies, and the number of studies included in each meta-analysis was not large, which may have led to strong heterogeneity in the results.
In summary, this study verified the accuracy of the results of previous studies to improve the homogeneity of future meta-analyses. There is a certain correlation between the brain imaging results of patients with HE and their cognitive function. Imaging means can be used to measure the cognitive function of patients with liver cirrhosis to detect the occurrence of HE as early as possible and achieve early diagnosis and treatment. During mild HE, it can prevent the progression of HE and reduce the burden of the disease. However, the number of relevant studies is insufficient to identify the best brain subregion for diagnosing HE, and more research is needed to explore the relationship between the impact of more brain regions and cognitive function in patients with HE.