1. Background
According to the global cancer statistics 2020, there are 905,677 new cases of liver cancer and 830,180 deaths per year (1). The morbidity and mortality rates associated with hepatocellular carcinoma (HCC), the sixth most common cancer and the fourth leading cause of cancer death worldwide (2, 3) are higher in China compared to other countries. The main risk factors of HCC mainly occur in the setting of cirrhosis and chronic hepatitis C in Western countries, but viral hepatitis B infection and aflatoxin uptake in China.
The potential curative therapies for early HCC include surgery, liver transplantation, and radiofrequency ablation, but these are effective only in one-third of HCC patients (4, 5). Multifocal and intermediate-stage HCC patients were treated with transcatheter arterial chemoembolization (TACE), and those patients who failed TACE or presented with more advanced HCC eventually received systematic treatment (6). Patients with advanced liver cancer treated with sorafenib, the only approved targeted drug, had a median overall survival (OS) of 10.9 months as compared with the placebo group, with an OS of 2.8 months in the past decade (7). Before 2017, no second‐line therapies had been approved for HCC patients who progressed with sorafenib, either participated in a clinical trial and continued to use sorafenib while imaging progression, or received the best supportive treatment. Recently, the survival rate of HCC patients has not been ideal although several second-line drugs have been used, including regorafenib, cabozantinib, and ramucirumab. Furthermore, the checkpoint inhibitors CheckMate-040 for Nivolumab and Keynote-240 for pembrolizumab as a second-line treatment of HCC have been approved by the FDA due to their sustained response in phase II clinical trials (8, 9). However, the phase III clinical study data of these two checkpoint inhibitors did not show any survival benefit, and the European Medicines Agency (EMA) has not yet approved any drug for HCC patients.
At present, little research for advanced HCC has been reported on combination therapy with immune checkpoint inhibitors and targeted drugs in the second or third-line treatment. However, the data related to the first-line treatment, including atezolizumab plus bevacizumab (NCT03434379) as well as pembrolizumab plus Lenvatinib (NCT03006926), showed good objective response rate (ORR) for unresectable HCC (4, 10). There are a few studies on the treatment of HCC with a combination of Nivolumab and Lenvatinib. Study117 is the only study on the first-line treatment of unresectable HCC with Nivolumab and Lenvatinib. Furthermore, a few other studies have also reported the treatment of HCC with Nivolumab and Lenvatinib, let alone the treatment of recurrent.
2. Objectives
In this study, we recruited 28 patients with recurrent HCC and treated them with Nivolumab combined with Lenvatinib. The objective of our study was aimed to provide a further therapeutic basis for PD-1 combined with multi-kinase targeted therapy in recurrent HCC after TKI treatment.
3. Methods
3.1. Patients
Twenty-eight patients with recurrent HCC were confirmed by the postoperative pathological examination or based on the European Association for the Study of the Liver (EASL) criteria (4) in Hunan Provincial People's Hospital from December 2016 to March 31, 2020. The inclusion criteria were as follows: (1) The HCC recurrence was confirmed by imaging; (2) patients with unresectable HCC progression after first-line treatment; and (3) patients with Child-Pugh class A. The exclusion criteria included: (1) HCC with portal vein tumor thrombus (PVTT) involving the main portal vein; (2) HCC with portal vein tumor thrombus (PVTT) involving the main portal vein; (3) patients with a history of secondary malignancy; (4) participants with severe renal disease, or significant cardiovascular problems. The study was approved by our hospital ethics committee. Written informed consent was conducted and obtained from all enrolled subjects before treatment.
Formula
3.2. Intervention
Patients received Nivolumab plus Lenvatinib as a salvage treatment after failure of first-line or even multi-line treatment. Nivolumab (3 mg/kg) and Lenvatinib (8 mg/day) were administered after every three weeks. The adverse events are assessed according to the Common Terminology Criteria for Adverse Events version 4.0 developed by the National Cancer Institute in the United States. If a patient experiences grade 3 - 4 adverse events as defined by the criteria, treatment is suspended. Treatment is continued with a half dose if the patient’s grade decreases to 2 or below. If the patient’s grade does not decrease, treatment is discontinued.
3.3. Outcome
Demographic and clinical statistics were collected, including age, sex, ECGO, High HBV, AFP levels, and previous treatment line number. Moreover, the dates of initiation and termination of the combination therapy were recorded until death or final contact. Progression-free survival (PFS) and OS were calculated from the beginning of the use of Nivolumab plus Lenvatinib.
3.4. Statistical Analysis
SPSS17.0 software and GraphPad Prism were used to perform all statistical analyses, including Kaplan-Meier survival curves. Data were expressed by median (range) according to distribution, which was confirmed by Kolmogorov-Smirnov analysis. Logistic regression was performed for risk factors of survival.
4. Results
4.1. Baseline Patient Characteristics
All patients were treated with Nivolumab and Lenvatinib combination at the time of radiological progression and received targeted therapy. As shown in Table 1, the baseline characteristics and follow-up information were summarized.
| Variables | Number |
|---|---|
| Age | 53.5 (32 - 69) |
| Sex, No. (%) | |
| Male | 16 (57.1) |
| ECGO | 1 (0 - 2) |
| High HBV, No. (%) | 6 (21.4) |
| AFP > 400 ng/mL, No. (%) | 21 (75.0) |
| Extrahepatic metastases | 1 (0 - 5) |
| Intrahepatic disease > 50%, No. (%) | 15 (53.6) |
| EBRT, No. (%) | 24 (85.7) |
| Previous treatment line number | 2 (1 - 3) |
| N + L therapy time (m) | 5.71 (2.3 - 21.3) |
| Follow-up time (m) | 8.7 (3.7 - 36) |
| OS (m) | 8.7 (3.7 - 36) |
| PFS (m) | 5.7 (2.1 - 36) |
| Death, No. (%) | 19 (67.9) |
During the beginning of the combination therapy, the patients (median: 53.5 years, range: 32 - 69 years, female/male: 12/16) were diagnosed with BCLC stage C and Child-Pugh grade A with portal vein tumor thrombus (PVTT) and chronic hepatitis B virus (HBV) 6 patients (21.4%) had high HBV copy numbers and 21 patients (75.0%) had high levels of alpha-fetoprotein (AFP). Seventeen patients (60.1%) had extrahepatic organ metastasis. Fifteen of them had extensive intrahepatic diseases, with >50% involvement. Twenty-four patients (85.7%) underwent external beam radiation (EBRT) for PVTT. 11 patients 39.3%) were treated with sorafenib only, and 17 patients (60.7%) were treated with at least two lines of multi-target treatment. At the initiation of therapy, 22 patients (78.6%) were treated with the combination of Nivolumab and Lenvatinib, who had an ECOG score of 1, and 6 patients (21.4%) showed an ECOG score of 2. None of the patients underwent genetic testing.
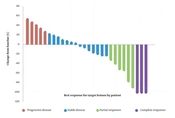
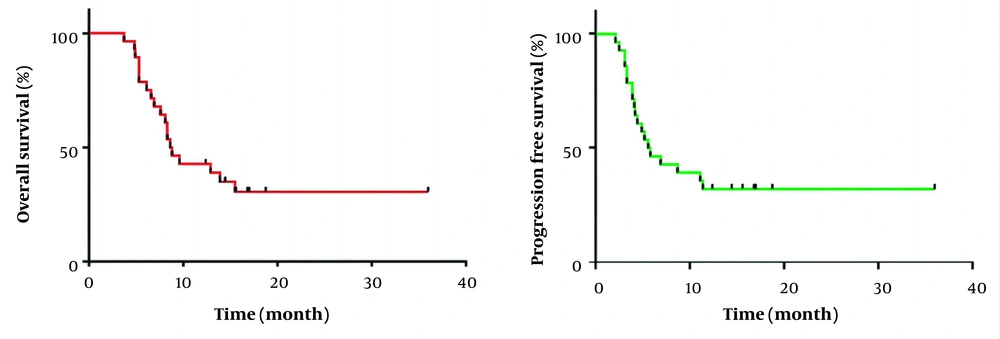
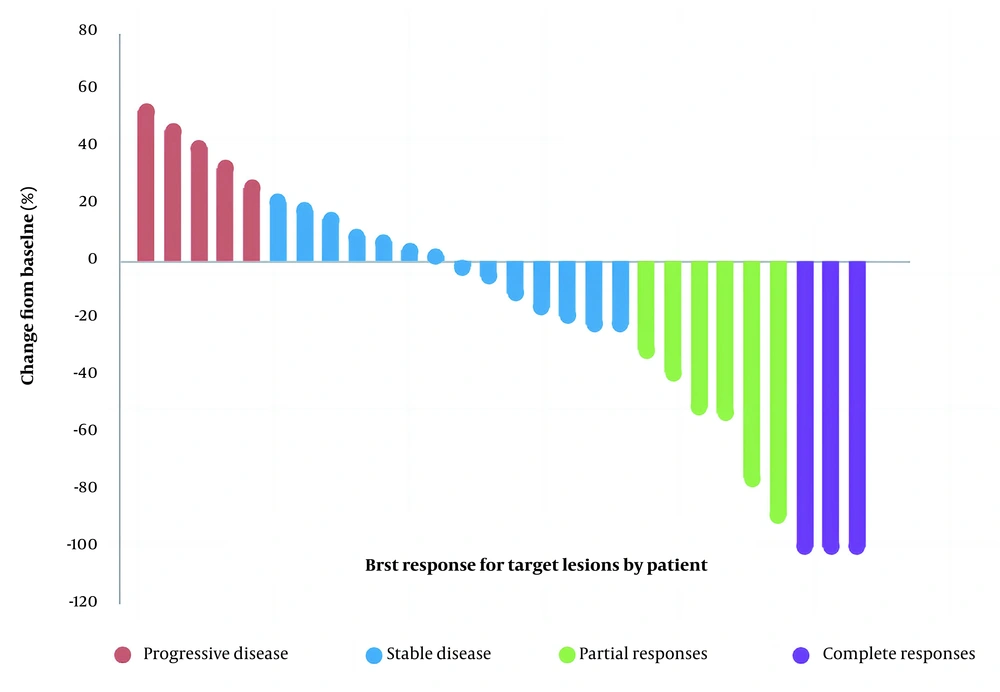
The best imaging outcome showed complete remission in 3 patients, stability in 20, and progression in 5 patients. The disease control rate (CR + PR + SD) was 23/28 (82.1%), with a median OS of 8.7 (3.7 - 36) months and a median PFS of 5.7 (2.1 - 36) months (Figure 1). In addition, a waterfall plot of tumor size was illustrated in Figure 2 for all patients: 5 patients experienced progressive disease (PD), 14 patients had stable disease (SD), 6 patients showed a partial response (PR), and 3 patients achieved completely disappeared (CR).
4.2. Clinical Imaging Characteristics of the Patients
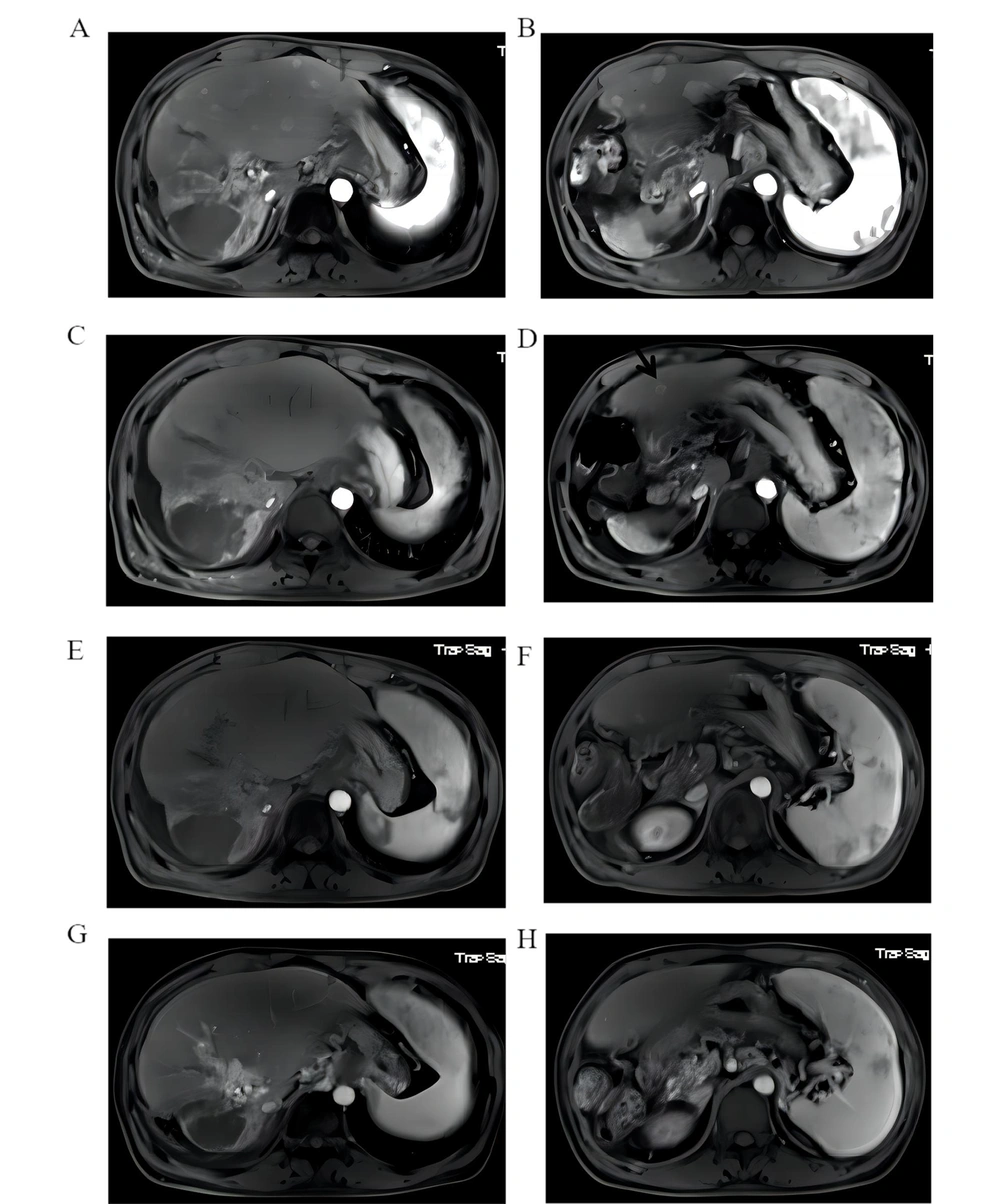
Patient No. 2 was diagnosed with HCC on December 2, 2017, and had PVTT and an initial BCLC stage of C. The patient received TACE twice and was then treated with sorafenib. AFP levels decreased from 281.1ng/mL to normal. On March 22, radiotherapy with a dose of 50Gy/25F for PVTT was performed, and the use of sorafenib was continued. In December 2018, an abdominal MRI examination revealed multiple metastatic lesions in the liver (Figure 3A and B). On the Patient's request, the combination of Nivolumab (180 mg every 3 weeks) and Lenvatinib (8 mg per day) was administered on December 12, 2018. MRI examination performed in February 2019 showed a significant reduction of intrahepatic lesions, with only S4 segment of liver lesions of 11mm diameter left (Figure 3C and D black arrow). MRI in June 2019 indicated no development of lesions in the S4 segment of the original liver, suggesting complete remission of the tumor (Figure 3E and F). After that, the MRI was repeated after every two months, and no new lesions were observed. The patient was treated with 15 cycles (affected by COVID-19) for 15.6 months, and imaging indicated complete remission at 9.6 months without any adverse side effects (Figure 3G and H).
An abdominal MRI examination revealed multiple metastatic lesions in the liver (A and B). MRI examination performed in February 2019 showed a significant reduction of intrahepatic lesions, with only S4 segment of liver lesions of 11mm diameter left (C and D black arrow). MRI in June 2019 indicated no development of lesions in the S4 segment of the original liver, suggesting complete remission of the tumor (E and F). The patient was treated with 15 cycles (affected by COVID-19) for 15.6 months, and imaging indicated complete remission at 9.6 months without any adverse side effects (G and H).
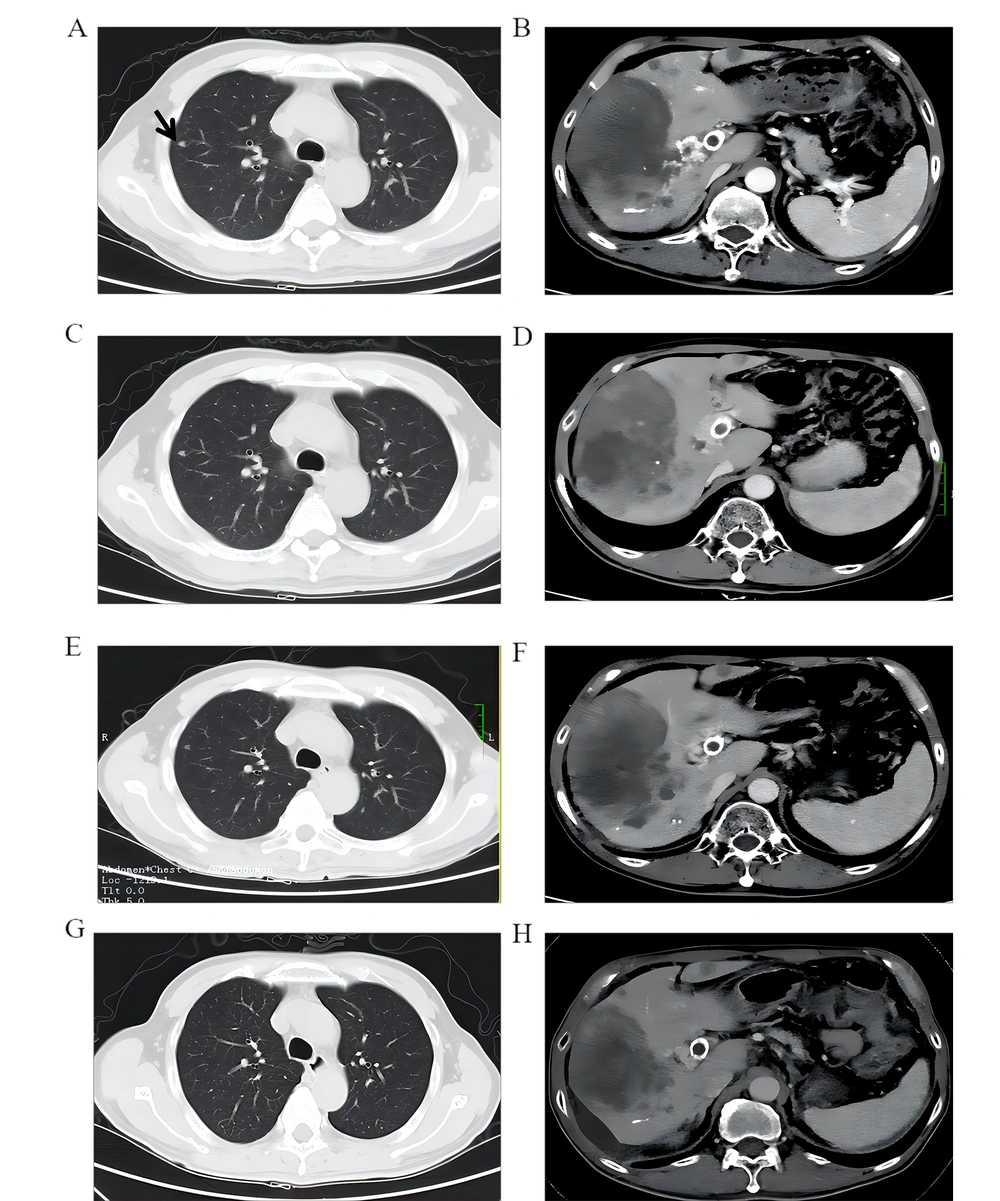
Patient No. 6 was diagnosed with HCC on June 8, 2018. MRI was not conducted because of portal vein stent installation. TACE was performed three times, and treatment with 8 mg/day Lenvatinib was started in October 2018. Chest and abdominal CT examination, performed on January 8, 2019, indicated a massive tumor in the right lobe of the liver with multiple intrahepatic metastases, extensive portal vein thrombosis, and right lung metastasis (Figure 4A and B). A total of 11 cycles of combined treatment were completed with Nivolumab (180 mg/3 weeks) plus Lenvatinib (8 mg/day) started on January 28, 2019. During the treatment, CT scans of the whole chest and abdomen performed every two months showed improvements, indicating that the condition was stable (Figure 4C, D, E, and F). The latest CT scan was done in February 2020, indicating ascites, partial tumor enlargement in the liver, and partial tumor shrinkage (Figure 4G and H). The patient had grade 2 diarrhea and grade 1 liver dysfunction during the treatment, and the patient returned to normal after treatment with glucocorticoid and supportive treatment.
Chest and abdominal CT examination, performed on January 8, 2019, indicated a massive tumor in the right lobe of the liver with multiple intrahepatic metastases, extensive portal vein thrombosis, and right lung metastasis (A and B). CT scans of the whole chest and abdomen performed every two months showed improvements, indicating that the condition was stable (C, D, E, and F). The latest CT scan was done in February 2020, indicating ascites, partial tumor enlargement in the liver, and partial tumor shrinkage (4G and H).
4.3. Adverse Reactions
All adverse reactions (AEs) are described in Table 2. Among the cohort of 28 patients, 18 patients developed grade 1 hyperbilirubinemia, 13 patients returned to normal after interrupted medication and nursing support, and 5 patients failed to continue treatment due to continuous increase of bilirubin levels due to liver tumor progression. Five patients developed grade 2 diarrhea and returned to normal after treatment with glucocorticoids. Nine patients developed hypertension of grade 2. None of the patients exhibited grade 3 or 4 side effects.
| Adverse Event | Grade 1 - 2 | Grade 3 - 5 |
|---|---|---|
| Fatigue | 0 | 0 |
| Rash | 0 | 0 |
| Lymphopenia | 0 | 0 |
| Hypertension, No. (%) | 9 (32.14) | 0 |
| Diarrhea, No. (%) | 5 (17.86) | 0 |
| Hyperbilirubinemia (64.29%) | 18 | 0 |
| Hypothyroidism | 0 | 0 |
| Hemorrhage | 0 | 0 |
4.4. Risk Factors for Survival
Furthermore, the study of the correlation between patient survival and clinical features of liver cancer is essential. The risk variables for survival were calculated using binary logistic regression analysis. It was found that Intrahepatic disease > 50% (OR = 46.181, 95%CI 1.062~2007.265, P = 0.046, Table 3) was the risk factor for survival of HCC patients.
| Variables | Wald | Odds Ratio | 95% CI | P |
|---|---|---|---|---|
| Age | 2.208 | 1.216 | 0.940~1.573 | 0.137 |
| Sex | 0.261 | 2.321 | 0.092~58.705 | 0.610 |
| ECGO | 2.548 | 0.039 | 0.001~2.096 | 0.110 |
| High HBV | 0.045 | 0.664 | 0.015~28.799 | 0.831 |
| AFP > 400 ng/mL | 0.880 | 0.175 | 0.005~6.690 | 0.348 |
| Extrahepatic metastases | 0.231 | 0.723 | 0.193~2.710 | 0.631 |
| Intrahepatic disease > 50% | 3.966 | 46.181 | 1.062~2007.265 | 0.046 |
| EBRT | 0.509 | 6.789 | 0.035~1310.433 | 0.476 |
| Previous treatment line number | 0.991 | 0.594 | 0.213~1.655 | 0.319 |
| N + L therapy time | 1.710 | 3.195 | 0.560~18.215 | 0.191 |
5. Discussion
A REFLECT study, a phase 3 open-label, multicenter noninferiority trial, showed the efficacy of a multi-kinase inhibitor, Lenvatinib, which was published in the Lancet journal (11). Patients treated with Lenvatinib, which met the study's primary criteria of non-inferiority, had longer median OS (13.6 months) than sorafenib (12.3 months). In August 2018, it was approved by the FDA as a first-line treatment drug for patients with unresectable advanced HCC. More importantly, the REFLECT trial excludes patients with bile duct invasion, main PVTT, or more than 50 % intrahepatic lesions.
Checkmate 040 is a multi‐center, open‐label, phase I/II study in which Nivolumab, as a complete human immunoglobulin G4 immune checkpoint inhibitor antibody in several cancers, was used for advanced HCC for both sorafenib‐naïve and ‐experienced patients. And the results showed only 29% (sorafenib‐naïve) and 18% (sorafenib‐experienced) patients demonstrated grade 3 - 4 AEs after Nivolumab treatment, indicating it was a safe and well-tolerated drug. In addition, as a second-line treatment for advanced HCC, Nivolumab was granted by the FDA but not EMA or several other global agencies (7). Similarly, an open-label and phase III clinical trial CheckMate 459 is also used to evaluate the efficacy of head-to-head Nivolumab versus sorafenib for advanced HCC patients as the first-line treatment. However, this study did not reach the intended primary endpoint; that is, Nivolumab did not exhibit an improvement in OS when compared to sorafenib (12).
Basic studies have shown that vascular endothelial growth factors can inhibit the immune function of the tumor, and anti-angiogenic drugs might locally improve the immune microenvironment inhibition of the tumors, suggesting a synergistic effect of anti-angiogenic drugs and immune checkpoint inhibitors (13). IMbrave150 is an open-label, phase 3 clinical trial for unresectable HCC patients to evaluate the efficacy of atezolizumab + bevacizumab versus sorafenib, which revealed decreased hazard ratio of death treated with atezolizumab-bevacizumab (OS rate at 12 months: 67.2%, median PFS: 6.8 months) when compared with sorafenib (OS rate at 12 months: 54.6%, median PFS: 4.3 months) (14). Our results showed that the OS rate at 12 months was 42.8%, the median OS of 8.7 (3.7 - 36) months, and the median PFS of 5.7 (2.1 - 36) months. A phase Ib study KEYNOTE 524 expanding the sample size was reported at ASCO Congress in 2020, which evaluated the use of Lenvatinib in combination with pembrolizumab for unresectable HCC, and the resulted showed that ORR, DCR, and mPFS were 46%, 88%, and 9.3 months, respectively (15). There were only a few HCC cases that underwent treatment with Nivolumab plus Lenvatinib (16). One ongoing open-label phase Ib study, Study117, was the only study that used Nivolumab combined with Lenvatinib in the treatment of unresectable HCC. Preliminary results of the American Society of Clinical Oncology Gastrointestinal Cancers Symposium 2020 ASCO (GI) included 30 patients, wherein 17 cases were of BCLC-B, and 13 cases were of BCLC-C. According to the study on salvage treatment for recurrent HCC, Kim et al. confirmed that 66 patients (93%) achieved CR after proton beam therapy (17). Lou et al. found that after hypofractionated radiotherapy (HFRT), 22.7% of patients achieved CR, 73.3% achieved PR, 4.0% had SD, and there were no cases of PD (18). In our research, the results showed that there were 3 (10%) patients who achieved CR, 20 (66.7%) patients had a PR, ORR was found in 76.7% of patients, and the DCR was 96.7%.
At present, second-line treatments for advanced HCC mainly include regorafenib, cabozantinib, and ramucirumab. The emergence of these drugs has improved the dilemma of first-line resistance, but the ORR remains unsatisfactory. The phase III clinical data of second-line treatments for advanced liver cancer with Nivolumab and pembrolizumab showed no survival benefit. In Western countries, the third-line treatment of liver cancer is considered the best supportive therapy. In fact, due to the advent of immune drugs and targeted drugs, the survival time of HCC patients has increased. For patients with good liver function and physical fitness scores, targeted drugs combined with immunotherapy can be used.
For recurrent HCC, there is no research report on the combination of immune checkpoint inhibitors and targeted drugs used in second or third-line therapy. The literature on the combination therapy of Nivolumab and Lenvatinib to treat HCC is sparse, let alone after first-line or even multi-line treatment failure. The researchers in our center enrolled 6 patients with recurrent HCC from China, and all had a history of hepatitis B cirrhosis, Child-Pugh stadium A, and BCLC stage C. After first-line treatment or even multi-line treatment failure, all the patients received treatment with Nivolumab plus Lenvatinib. Patient NO.1 received sorafenib, apatinib, and axitinib successively, and patient NO.5 received sorafenib and Apatinib before the combination therapy of Nivolumab and Lenvatinib. HCC patients with PVTT are prone to liver metastasis and portal hypertension, and PVTT is one of the factors for the poor prognosis of HCC. Therefore, patients with PVTT are excluded from clinical studies such as REFLECT. In our cohort, all patients had PVTT, and the combination of Nivolumab and Lenvatinib achieved satisfactory curative effects (3 cases showed complete remission, and 20 cases were stable) in the absence of serious drug side effects. Several risk factors in all 28 patients were analyzed, and according to their experience in radiotherapy for PVTT, they had a longer survival rate. Patient No. 5, who was evaluated to have progressive disease, did not receive PVTT external irradiation. Patient NO.2, who was assessed to achieve complete remission, received PVTT external irradiation before the combination therapy of Nivolumab and Lenvatinib. The rest of the patients with stable disease received PVTT external irradiation during the combination therapy. Studies have proved that the complications caused by PVTT are considered a common cause of death in HCC patients. Therefore, radiotherapy with PVTT can prolong the survival rate and improve liver blood perfusion and liver function, thus providing a basis for systemic treatment (19). Whether early PVTT external irradiation intervention is more conducive to survival needs to be further explored.
The side effects of immunotherapy and targeted drugs are different from those of chemotherapy. HCC treatment with immune checkpoint inhibitors demonstrated a substantial increase in AST/ALT levels when compared with other solid tumors (20). Other common side effects included rash, lipase increase, and amylase increase (21). The side effects associated with Lenvatinib treatment mainly include hyperbilirubinemia, hypertension, and diarrhea, and the occurrence probability of grade 1 - 2 hyperbilirubinemia is 60%, and that of grade 3 - 4 hyperbilirubinemia is 20% among these patients. Furthermore, the rate of hyperbilirubinemia of the CP-B group was higher than that of the CP-A group (22).
The side effects associated with Nivolumab and Lenvatinib include liver function lesions; however, the effects of combination therapy on bilirubin are unknown. Among the 28 patients in our study, 18 patients developed grade 1 hyperbilirubinemia, 13 patients returned to normal after terminating the medication and nursing support, and 5 patients failed to continue the treatment due to a continuous increase in bilirubin because of liver tumor progression. In our study, 9 patients developed hypertension, which is considered a common side effect of Lenvatinib, and was cured by an anti-hypertensive drug. Both checkpoint inhibitors and Lenvatinib were associated with the side effects of diarrhea, which was reported in 5 patients in this study and treated with glucocorticoid therapy. In general, the side effects associated with combination therapy of Nivolumab and Lenvatinib on patients in the treatment of HCC were controllable, and no grade 3 - 4 side effects were observed.
However, there are still several limitations demonstrated as follows: Firstly, this is a retrospective study, and bias in patient selection could exist. Secondly, a larger sample size was needed for further investigation.
As a salvage treatment of patients with advanced HCC, the practical experience of Nivolumab and Lenvatinib combination after first-line or even multi-line treatment was proved to be efficacious and safe.