1. Introduction
Hepatitis E virus (HEV) infection is an enterically transmitted acute viral hepatitis, which presents as large-scale epidemics and sporadic infections in endemic areas (1). HEV is the most common cause of acute viral hepatitis in the world (2, 3). HEV genotypes 1 and 2 are responsible for large outbreaks and sporadic cases in the developing countries and are usually waterborne. HEV genotypes 3 and 4 are zoonotic infections and cause sporadic cases of foodborne HEV infection in Europe and North America, which are mainly associated with the consumption of meat or offal from of wild or domestic swine (4). HEV infection usually takes a clinically silent course in the majority of patients (5). However, a few subjects may develop a more severe course, particularly pregnant females or patients with underlying chronic liver diseases (6). In contrast to epidemic HEV infection, autochthonous HEV infection has higher disease rates among males and older adults, and often presents with less severe disease (7). Extrahepatic complications of HEV infection include neurological manifestations such as Guillain-Barré syndrome, thrombocytopenia, hemolytic anemia, glomerulonephritis, cryoglobulinemia, myocarditis, thyroiditis, and meningitis (8). In addition, HEV is recently described as a causative agent of acute pancreatitis. However, clinically overt pancreatitis is only reported in association with cases of fulminant hepatic failure from centers in South Asia, where genotype 1 prevails (9-11). The current study reports the case of an 85-year-old male with autochthonous acute HEV infection, which progressed into acute-on-chronic liver failure (ACLF) and was complicated by severe acute pancreatitis.
2. Case Report
An 85-year-old male was admitted to the department of hepatology due to new-onset jaundice with dark colored urine and bright stool. The patient reported a lack of appetite and 3 kg loss of weight in the absence of abdominal pain and fever. There was no history of alcohol abuse, gallstone disease, and no obvious attacks of pancreatitis. The patient did not travel abroad and not received any transfusions. Physical examination revealed telangiectasia and conjunctival jaundice. One year ago the patient was referred to the department of hepatology, and compensated liver cirrhosis caused by steatohepatitis was diagnosed.
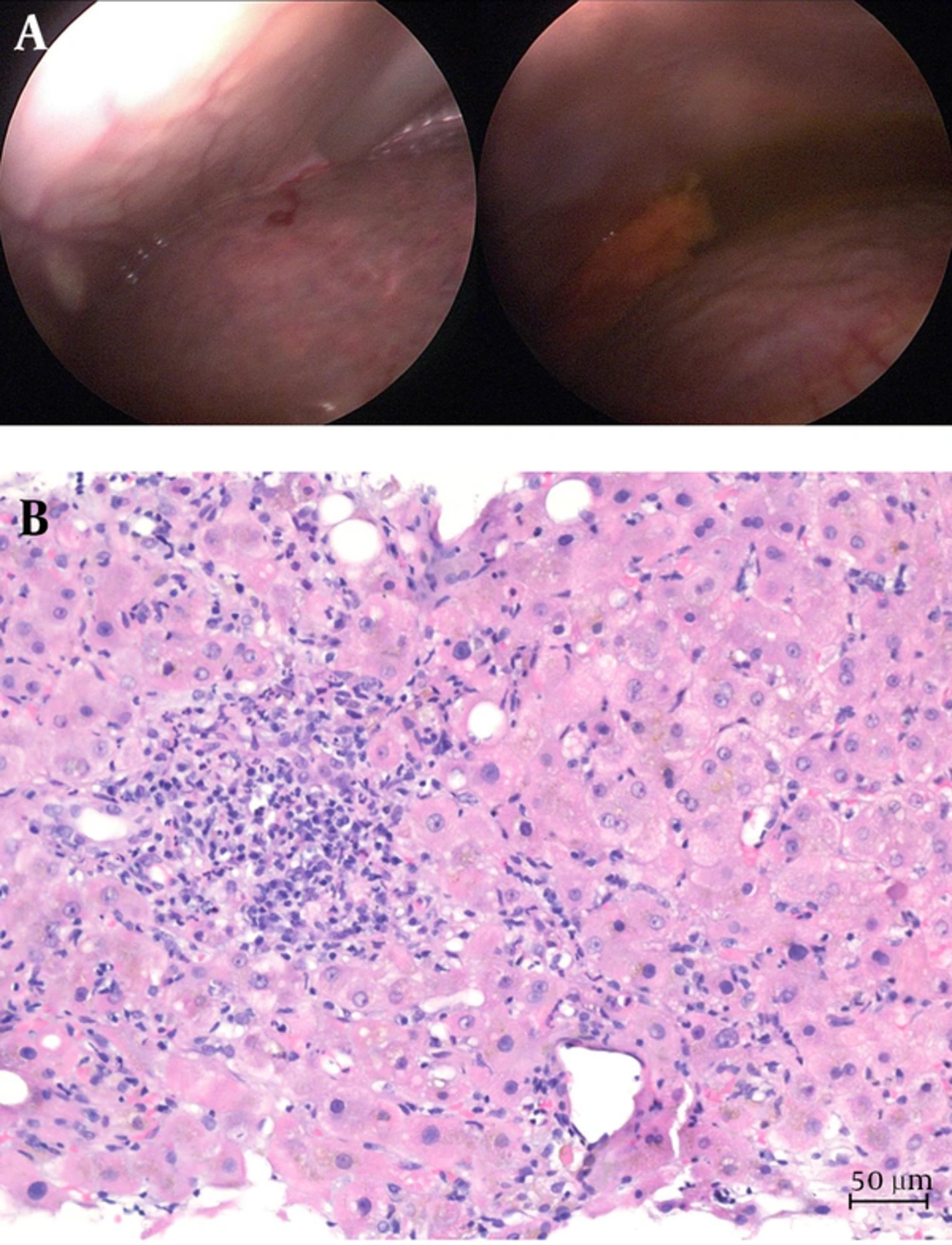
Laboratory work-up showed icteric hepatitis with increased total bilirubin (216 µmol/L, 10 × upper limit of normal (ULN), increased alanine aminotransferase (12.29 µmol/L/s, 16 × ULN), and aspartate aminotransferase (22.67 µmol/L/s, 39 × ULN) in the absence of significant alkaline phosphatase elevation (3.86 µmol/L/s, 1.9 × ULN). Serological tests for viral hepatitis A to C were negative. Serum levels for immunoglobulins G and A were elevated (IgG 19.8 g/L, 1.3 × ULN; IgA 9.02 g/L, 2.0 × ULN). Autoantibody testing for autoimmune hepatitis was negative. Abdominal ultrasound of the abdomen excluded bile stone disease and confirmed signs of cirrhosis. Portal vein and liver veins were regularly perfused. Endoscopic ultrasound showed a non-dilated common bile duct and pancreatic ducts but few calcifications of the pancreas. Mini-laparoscopy was performed on day 5 and confirmed liver cirrhosis with ascites (Figure 1A). Histological analysis confirmed acute hepatitis superimposed on cirrhosis (Figure 1B).
A, Representative Macroscopic Imaging of the Irregular Liver Surface Obtained During Mini-Laparoscopy; B, Hematoxylin and Eosin Stain Showing Acute Hepatitis Superimposed on Cirrhosis with Portoportal Fibrous Septae and Foci of lymphoplasmacellular Infiltrates as Well as Intra-Acinar Granulocytes, Activated Macrophages and Cholestasis (200X Magnification).
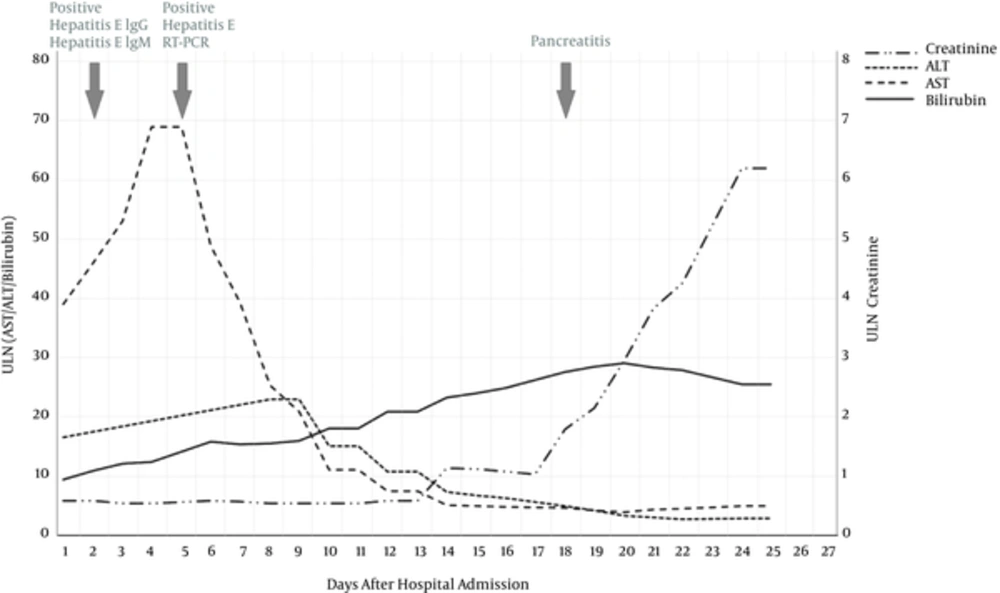
After obtaining the positive anti-HEV IgM antibodies on the day 2, HEV infection was confirmed by reverse transcription polymerase chain reaction (RT-PCR). Due to an improvement of transaminase levels, ribavirin was not initiated at this time. On day 14, the patient serum creatinine increased to 137 µmol/L in combination with total serum bilirubin of 433 μmol/L (21 × ULN) resulting in grade 1 ACLF according to European definitions (laboratory findings are shown in Table 1) (12). Four days later, the patient reported pain in the upper abdomen and bloating. Serum lipase markedly increased to 39.7 μmol/L/s (52 × ULN) and endoscopic ultrasound confirmed signs of acute edematous pancreatitis and bile stones were excluded again. Despite fluid resuscitation with albumin, renal function further declined and oliguria developed fulfilling the criteria for hepatorenal syndrome. At this time, the patient had developed grade 2 ACLF. Investigation of the ascitic fluid revealed increased neutrophils (3.34 × 103/µL), which defined bacterial peritonitis. As pancreatic ascites was unlikely (ascitic fluid lipase increased less than 3-fold), antibiotic treatment with piperacillin and tazobactam was initiated. Treatment with terlipressin did not improve renal function. On day 21, the patient developed dyspnea and respiratory failure (oxygenation index of 184), defining grade 3 ACLF. Repeated paracentesis showed non-response to empiric antibiotic treatment and negative bacterial culture results. Despite therapeutic efforts and escalation of antibiotic therapy (meropenem and linezolid) the patient died 28 days after hospital admission (Figure 2).
| Day | 1 | 4 | 6 | 10 | 14 | 18 | 21 |
|---|---|---|---|---|---|---|---|
| Creatinine, mg/dL | 0.80 | 0.74 | 0.80 | 0.74 | 1.56 | 2.44 | 5.81 |
| Total bilirubin, mg/dL | 12.64 | 15.33 | 19.42 | 22.11 | 28.55 | 33.81 | 34.16 |
| INR | 1.3 | 1.5 | 1.6 | 1.7 | 2 | 1.2 | n.d. |
| MAP, mmHg | 100 | 101 | 117 | 103 | 76 | 135 | 96 |
| Use of vasopressors | No | No | No | No | No | No | Yes |
| ACLF Grade | 0 | 0 | 0 | 0 | 1 | 2 | 3 |
| CLIF organ failure score | 7 | 8 | 8 | 8 | 9 | 9 | 12 |
| CLIF AD score | 56 | 57 | 61 | 60 | N/A | N/A | N/A |
| CLIF ACLF score | N/A | N/A | N/A | N/A | 57 | 61 | 72 |
Abbreviations: MAP: mean arterial pressure; N/A, not applicable n.d. not determined.
3. Discussion
The current case highlights autochthonous HEV infection as a cause of acute decompensation and ACLF in patients with preexisting liver disease and suggests a possible association with acute pancreatitis as an extrahepatic manifestation. Although acute pancreatitis is a well-recognized complication of viral hepatitis, the majority of cases are either associated with benign forms of acute viral hepatitis and resolve with little morbidity in conjunction with the recovery from hepatitis or with fulminant liver failure. The mechanism of pancreatic injury in viral hepatitis is unclear. Ham et al. studied 42 autopsied cases of acute liver failure predominantly due to viral hepatitis and found all grades of pancreatitis (13). In addition, chronic relapsing forms of pancreatitis in association with viral hepatitis are hypothesized (14). Proposed mechanisms include an edema of the ampulla vateri due to viral infection and in acute liver failure (15). Most cases of HEV-associated pancreatitis are associated with genotype 1 and reported from Southern Asia (8). In the current case, the patient did not travel to this region. In our center in Germany, genotype 3 prevails and genotyping was not performed in this particular case. As other causes of acute pancreatitis were ruled out, an association of pancreatitis with HEV infection was likely.
Severe courses of acute HEV infection are described in individuals with preexisting chronic liver disease (16), elderly males and pregnant females (17). Endemic HEV infection is recognized as one of the leading causes of acute decompensation of cirrhosis in the developing countries and is estimated to account for approximately 21% of ACLF in Asia and Africa (18). In contrast, autochthonous HEV infection usually presents with a milder course of disease (7) and is only sporadically reported as the cause of decompensation and ACLF in large Western cohorts (19). A retrospective cohort of patients treated with ribavirin for autochthonous HEV infection revealed that mortality occurred only in patients with acute decompensation of underlying cirrhosis (20). It cannot be excluded that other hepatic or extrahepatic factors might have contributed to the decompensation of cirrhosis in the current study patient (21). The time course and extent of hepatitis suggested HEV infection as the major decompensating event. Although hepatitis E-associated decompensation and ACLF developed before the onset of pancreatitis in the current case, acute pancreatitis most likely accelerated the course of disease by driving acute renal failure as one of the most relevant single organ failures in ACLF by European definition (12).
It remains elusive whether treatment with ribavirin would have ameliorated the course of ACLF and prevented acute pancreatitis as an extrahepatic manifestation. However, the severe course of HEV infection in elderly patients with underlying liver disease evidence supports the early use of ribavirin in patients at risk (20).
3.1. Conclusions
Despite its association with a benign course, even autochthonous HEV infection can precipitate ACLF and may cause severe extra hepatic manifestations in patients with underlying liver disease.