1. Background
Obesity remains a significant health issue in today’s healthcare landscape. Conventional treatments for obesity have not yielded efficient results, with bariatric procedures proving to be the only effective method for weight loss (1). The prevalence of metabolic diseases continues to increase, and one of its manifestations, known as nonalcoholic fatty liver disease (NAFLD), has significantly impacted liver health (2). The rising incidence of hepatic manifestations is closely related to the rising incidence of fatness (3, 4). It is known that NAFLD can progress to steatohepatitis, fibrosis, and cirrhosis (5). Over the past decade, bariatric/metabolic surgery has been recognized as an effective approach for the remission of obesity-related comorbidities (6).
Sleeve gastrectomy (SG), a common bariatric or metabolic surgery that involves the removal of a part of the stomach, is frequently performed to facilitate weight loss (6). The Roux-en-Y gastric bypass (RYGB) is widely recognized as the primary and preferred procedure in bariatric surgery (7, 8). This procedure, which is frequently regarded as the gold standard, is preferred by numerous bariatric surgeons due to its effectiveness in promoting long-term weight loss and alleviating obesity-related conditions among patients (9, 10).
Since 2001, the minigastric bypass (MGB) has been introduced as a potential alternative to RYGB (11-13). Minigastric bypass, which combines the merits of SG and RYGB, is a safe, simple, and effective surgery (11). In 2017, it constituted about 4.8% of bariatric and metabolic surgeries worldwide (6). Substantial evidence suggests that bariatric and metabolic surgeries contribute to improvements in NAFLD and liver function tests (LFTs). Furthermore, recent studies indicate that bariatric surgeries can significantly alleviate the histological and biochemical symptoms of NAFLD. Interestingly, improvements in liver steatosis and fibrosis appear to be more pronounced in Asian populations compared to non-Asian ones (14, 15). However, some patients experience deterioration in LFT and even liver failure following RYGB (16-18). Bariatric surgery seems to have the ability to regulate liver parenchymal enzymes, such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT). However, it remains uncertain whether the procedure offers an unequivocal benefit (19). The optimal assessment of the impact of bariatric surgery on NAFLD involves direct analysis of changes in liver histology. However, biopsy carries certain risks. The development of non-invasive methods to measure liver fibrosis and steatosis could prove beneficial, if accessible (20). The British Obesity and Metabolic Surgery Society (BOMSS) recommends LFT monitoring after bariatric surgery for the possibility of deterioration (21). Acute liver injury (ALI) and acute liver failure (ALF) are rare complications following bariatric surgery, and there is a lack of substantial evidence-based guidance (22). However, bariatric surgery remains the primary treatment for patients with NAFLD. Several studies suggest that alternative surgical procedures may be more effective in treating NAFLD compared to RYGB (23, 24).
Identifying the most effective surgical procedure for treating NAFLD and preventing the decline in liver function remains a challenge.
2. Objectives
Therefore, this study aimed to compare the postoperative recovery of NAFLD between RYGBP and MGB and to evaluate the risk factors associated with the deterioration of liver function.
3. Methods
This cross-sectional study used a prospectively collected database of 90 patients, aged over 18 years, with a Body Mass Index (BMI) ranging from 35 to 50 kg/m². The patients were equally divided into two groups of 45 each, with one group undergoing MGB and the other undergoing RYGB. The data was collected from multiple centers in Esfahan city (Shariati Hospital, Kashani Hospital, Alzahra Hospital, and Amin Hospital) between 2018 and 2021.
The exclusion criteria and contraindications for bariatric surgery were as follows: (1) patients who, for any reason, did not wish to participate in this study, (2) patients with a malignancy, (3) pregnant women, (4) patients with inflammatory bowel disease (or any life-threatening disease), and (5) patients with a BMI of more than 50 kg/m². Additionally, patients without two years of identifiable medical management, patients with an unstable psychiatric disorder, patients who were not expected to comply after evaluation and consultation for surgery, and patients who were unable to care for themselves or lacked social and family support were excluded.
In this cross-sectional study, patients were selected based on the type of bariatric surgical procedure they underwent (MGB or RYGB). The selection was made from a prospectively collected database of eligible patients aged over 18 years, all of whom had a BMI between 35 and 50 kg/m². The selection process involved a thorough review of medical records to identify patients who met the inclusion criteria. It is important to note that patients were not actively assigned to specific groups or interventions. Throughout the study, all ethical considerations were strictly adhered to. The study protocol, along with the procedures for informed consent, were reviewed and approved by the Islamic Azad University ethics committee (IR.IAU.NAJAFABAD.REC.1397.047).
3.1. Primary Objectives
The primary objective of this study was to compare the postoperative recovery of NAFLD between patients who underwent RYGBP and those who underwent MGB.
3.2. Secondary Objectives
The secondary objectives were as follows:
(1) To evaluate the risk factors contributing to the deterioration of liver function.
(2) To assess the influence of surgical procedures on hepatic markers and metabolic parameters.
(3) To identify and consider potential confounding variables (e.g., age and gender) and effect modifiers (e.g., baseline BMI).
Potential confounders, which were intricately interwoven within the framework of this research, included variables that could influence the relationship between surgical techniques and outcomes. These confounders, which needed to be considered in this study, included demographic factors, such as age and gender, the presence of comorbidities, baseline BMI, and the level of social and familial support available to patients. Effect modifiers, which were crucial in uncovering the nuances of this investigation, included factors that could alter the effect of surgical techniques on outcomes. In this study, we focused on the baseline BMI and age as potential effect modifiers, given their potential to significantly influence the outcomes observed.
Prior to all procedures, patients underwent a comprehensive preoperative assessment, which included a psychological evaluation, cardiopulmonary assessment, anesthetist review, endocrine evaluation, nutritional status assessment, and consultation with a bariatric counselor. Abdominal ultrasonography was performed to assess the grade of fatty liver, liver size, and presence of gallstones. An endoscopy of the upper gastrointestinal tract was also conducted to rule out any disease. Before the operation, we measured the patients’ height, weight, sagittal diameter, and blood pressure. We also collected 5 cc of venous blood from patients in both groups prior to the operation and at three and six months postoperatively to determine the serum levels of hepatic enzymes (alkaline phosphatase ALP, AST, and ALT), gamma-glutamyl transferase (GGT), hemoglobin, platelet count, mean corpuscular volume (MCV), acute phase reactants (ferritin and CRP), fasting blood sugar, and hemoglobin A1C.
Outcomes, which constituted a central aspect of this investigation, included a wide range of measurements, including the levels of hepatic enzymes, such as ALP, AST, ALT, and GGT. In addition, hemoglobin levels, platelet count, MCV, acute phase reactants (e.g., ferritin and CRP), fasting blood sugar, and hemoglobin A1C were measured.
Interventions:
3.3. Laparoscopic RYGB (LRYGB)
This procedure incorporated both malabsorption and restriction. We created a small pouch of approximately 30 mL by activating the stapler about 4 cm from the junction entering the lesser sac. The jejunum was then transected 50 cm distal to the duodenojejunal flexure. The distal divided end of the jejunum was tailored to a small gastric pouch, forming a gastrojejunostomy anastomosis. The proximal divided end of the jejunum was tailored to the jejunum, forming a jejunojejunostomy anastomosis. This procedure resulted in the creation of two limbs. The Roux limb was about 150 cm in length, while the biliopancreatic limb was approximately 50 cm long.
3.4. Mini Gastric Bypass Procedure
The MGB procedure, while being mildly restrictive, primarily relied on malabsorption. Essentially, we created a narrow gastric tube. We initially located the crow’s foot on the minor gastric curvature of the stomach. We then divided the stomach with a stapler, moving upwards parallel to the minor curvature. For calibration, we used a 38-Fr orogastric tube/bougie. The next step involved creating a loop of jejunum 200 cm from the Treitz ligament, which was then tailored to form the biliopancreatic limb (BPL) in an antecolic fashion. This comparative analysis relied on the exposures and predictors, embodied by two distinct surgical techniques, that is, LRYGB and MGB.
For statistical analysis, we carefully managed quantitative variables through normalization and addressed missing data as necessary. We used descriptive statistics to summarize continuous data and made comparisons between the surgical technique groups using suitable tests, while considering the distribution of data. For categorical variables, we used frequency tables and chi-square tests to evaluate associations. To account for potential confounders, we adjusted for relevant covariates in the multivariate models. The significance level was set at P < 0.05. All statistical analyses were conducted using SPSS Version 24. We employed these methods meticulously to ensure the robustness and reliability of our findings.
We addressed the issue of missing data by using the imputation technique for certain individuals with incomplete information. Imputation is a process that estimates missing values based on the data that is available. It is a common method for dealing with missing data in statistical analyses. After the missing values were imputed, we carried out a thorough bias assessment to evaluate how these imputed values might affect the findings of our study. This assessment involved checking whether the imputed values introduced any significant bias into our results by comparing them with the original observed values. Conducting such bias assessments is crucial to ensure the validity and reliability of the conclusions drawn from our data, even after addressing missing data through imputation.
4. Results
The study included a total of 90 patients. Half of the participants (n = 45) underwent RYGB, including 33 (73.3%) female and 12 (26.7%) male patients. The mean age of patients in this group was 39.2 years, with a mean BMI of 47.3 kg/m2 and a mean sagittal diameter of 124.8. The other half (n = 45) underwent MGB, including 35 (77.8%) female and 10 (22.2%) male patients. The mean age of patients in this group was 38.1 years, with a mean BMI of 47.5 kg/m2 and a mean sagittal diameter of 123.5. The study found no significant difference in age, BMI, or gender prior to the operation between patients who underwent RYGB and those who underwent MGB (P = 0.87). In both groups, the patients were mostly between 30 and 45 years of age (approximately 50.0% of both groups) Table 1.
| Variables | RYGB | MGB | ALL | P-Value |
|---|---|---|---|---|
| Patient (n) | 45 | 45 | 90 | |
| Age (y) | 39.2 ± 10.9 | 38.1 ± 9.9 | 38.6 ± 10.4 | 0.644 |
| Sex (male/ female) | 12/33 (27.6/73.3) | 10/35 (22.2/77.8) | 22/68 (24.4/75.6) | 0.624 |
| Sagittal diameter (cm) | 124.8 ± 15.3 | 123.5 ± 16.3 | 124.15 ± 15.8 | 0.691 |
| BMI (kg m-2) | 47.3 ± 3.1 | 47.5 ± 4.1 | 47.4 ± 3.6 | 0.818 |
a Values are expressed as (%) or Mean ± SD.
The status of the preoperative laboratory and clinical results at baseline is presented in Table 2. In both groups, there were no significant differences in these variables between patients treated with MGB and those treated with RYGB. According to the P-values, no significant differences were observed between the two groups. Prior to surgery, more than 90% of patients were diagnosed with NAFLD based on their ultrasonographic pattern, and no significant differences were found between the two groups in this regard Table 2.
| Variables | RYGB | MGB | P-Value |
|---|---|---|---|
| Systolic blood pressure (mmHg) | 116.2 ± 15.6 | 117.4 ± 13.0 | 0.714 |
| Diastolic blood pressure (mmHg) | 78.4 ± 11.2 | 77.7 ± 8.7 | 0.724 |
| Hb (g/L) | 12.8 ± 2.4 | 13.5 ± 2.7 | 0.226 |
| MCV (fL) | 85.4 ± 10.2 | 84.1 ± 10.5 | 0.559 |
| Plt (× 10 9/L) | 229.3 ± 113.6 | 254.5 ± 111.5 | 0.291 |
| Glucose (mmol/L) | 129.6 ± 86.9 | 134.2 ± 81.8 | 0.798 |
| HbA1c (mmol/mol) | 6.5 ± 1.3 | 6.4 ± 1.6 | 0.674 |
| CRP (mg/L) | 9.8 ± 5.8 | 10.1 ± 4.8 | 0.785 |
| Ferritin (µg/L) | 222.7 ± 168.0 | 250.1 ± 179.3 | 0.456 |
| AST (µg/L) | 23.7 ± 17.8 | 22.5 ± 15.1 | 0.736 |
| ALT (µg/L) | 28.8 ± 19.4 | 27.1 ± 19.9 | 0.682 |
| ALK (µg/L) | 82.3 ± 60.8 | 85.1 ± 63.9 | 0.833 |
| GGT (µg/L) | 30.8 ± 27.4 | 28.2 ± 19.1 | 0.613 |
a Values are expressed as Mean ± SD.
Regarding the postoperative posture status, both patient groups experienced a significant reduction in BMI, weight, and sagittal diameter (P = 0.05). During the first three months of follow-up, no significant differences were observed between the groups in terms of BMI, weight, or sagittal diameter (P > 0.05). However, a significant decrease in BMI and weight was observed in patients who underwent MGB within the six-month period (P = 0.000 for BMI and P = 0.003 for weight) Table 3.
| Kind of Operation | Time | P-Value | ||||
|---|---|---|---|---|---|---|
| Pre-operation | After 3 Months | After 6 Months | Compare of Pre-operation and After 3 Months | Compare of After 3 Months and After 6 Months | Compare of Pre-operation and After 6 Months | |
| Weight (Kg) | ||||||
| RYGB | 4.13 ± 8.127 | 9.12 ± 3.113 | 2.12 ± 6.103 | 000.0 | 000.0 | 000.0 |
| MGB | 5.12 ± 4.129 | 0.12 ± 5.112 | 1.12 ± 8.95 | 000.0 | 000.0 | 000.0 |
| P-Value | 572.0 | 762.0 | 003.0 | |||
| BMI (kg m-2) | ||||||
| RYGB | 1.3 ± 3.47 | 7.2 ± 9.41 | 9.2 ± 3.38 | 000.0 | 000.0 | 000.0 |
| MGB | 0/4 ± 5.47 | 5.3 ± 3.41 | 8.2 ± 1.35 | 000.0 | 000.0 | 000.0 |
| P-value | 818.0 | 355.0 | 000.0 | |||
| Sagittal diameter (cm) | ||||||
| RYGB | 3.15 ± 8.124 | 7.14 ± 7.118 | 9.13 ± 0.110 | 000.0 | 000.0 | 000.0 |
| MGB | 3.16 ± 5.123 | 8.16 ± 4.116 | 0.17 ± 4.106 | 000.0 | 000.0 | 000.0 |
| P-value | 691.0 | 495.0 | 273.0 | |||
a Values are expressed as Mean ± SD.
After three months, only the RYGB group showed significant improvements in the AST and ALT levels, while the MGB group did not show any significant improvement (P > 0.05). There was no significant difference between the two groups during the six-month follow-up period. Significant changes were observed in the AST and ALT levels after RYGB from the preoperative stage to the three-month follow-up and from the three-month follow-up to the six-month follow-up (P = 0.05). However, the changes in the MGB group were not as significant (P > 0.05). After six months, the RYGB group showed a significant improvement in ALK values, unlike the MGB group. The level of GGT was reported to be significant in the MGB group before and three months after surgery, and the GGT level in the MGB group was more noticeable than in the RYGB group Table 4.
| Kind of Operation | Time | P-Value | ||||
|---|---|---|---|---|---|---|
| Pre-operation | After3 Months | After6 Months | Compare of Pre-operation and After 3 Months | Compare of After 3 Months and After 6 Months | Compare of Pre-operation and After 6 Months | |
| AST (µg/L) | ||||||
| RYGB | 23.7 ± 17.8 | 30.8 ± 18.5 | 24.4 ± 17.3 | 0.000 | 0.000 | 0.171 |
| MGB | 22.5 ± 15.1 | 23.5 ± 15.8 | 22.2 ± 14.8 | 0.372 | 0.313 | 0.703 |
| P-value | 0.736 | 0.048 | 0.537 | |||
| ALT (µg/L) | ||||||
| RYGB | 28.8 ± 19.4 | 36.9 ± 20.8 | 28.7 ± 19.0 | 000/0 | 000/0 | 0.713 |
| MGB | 27.1 ± 19.9 | 28.0 ± 19.2 | 26.7 ± 18.3 | 0.104 | 0.178 | 0.652 |
| P-value | 0.682 | 0.038 | 0.617 | |||
| ALK (µg/L) | ||||||
| RYGB | 82.3 ± 60.8 | 89.5 ± 49.3 | 115 ± 57.4 | 0.078 | 0.000 | 0.000 |
| MGB | 85.1 ± 63.9 | 84.0 ± 60.8 | 88.4 ± 51.9 | 0.755 | 0.165 | 0.475 |
| P-value | 0.833 | 0.637 | 0.022 | |||
| GGT (µg/L) | ||||||
| RYGB | 30.8 ± 27.4 | 27.2 ± 26.0 | 24.5 ± 23.5 | 0.515 | 0.110 | 0.229 |
| MGB | 28.2 ± 19.1 | 24.2 ± 18.6 | 149.7 ± 14.7 | 0.320 | 0.011 | 0.038 |
| P-value | 0.613 | 0.538 | 0.249 | |||
a Values are expressed as Mean ± SD.
After six months, no significant differences were observed in the systolic and diastolic blood pressure, blood platelet count, hemoglobin, or MCV in either group. During the six-month follow-up, both groups showed a significant decrease in glucose and HbA1c levels, with no significant difference between the groups. The ferritin and CRP levels showed a greater increase in the RYGB group than in the MGB group three months after surgery Table 5.
| Variables | Baseline | 3 Months | 6 Months | |||
|---|---|---|---|---|---|---|
| RYGB | MGB | RYGB | MGB | RYGB | MGB | |
| Systolic blood pressure (mmHg) | 116.2 ± 15.6 | 117.4 ± 13.0 | 116.1 ± 16.2 | 118.0 ± 13.2 | 115.4 ± 16.4 | 117.1 ± 13.6 |
| Diastolic blood pressure (mmHg) | 78.4 ± 11.2 | 77.7 ± 8.7 | 78.8 ± 11.1 | 76.9 ± 9.0 | 78.2 ± 12.4 | 76.2 ± 9.9 |
| Hb (g/L) | 12.8 ± 2.4 | 13.5 ± 2.7 | 12.8 ± 2.4 | 12.8 ± 3.0 | 12.8 ± 3.1 | 13.4 ± 2.8 |
| MCV (fL) | 85.4 ± 10.2 | 84.1 ± 10.5 | 83.9 ± 10.5 | 83.0 ± 10.4 | 85.5 ± 10.9 | 86.1 ± 10.7 |
| Plt (× 109/L) | 229.3 ± 113.6 | 254.5 ± 111.5 | 234.3 ± 121.5 | 249.4 ± 111.1 | 222.9 ± 114.2 | 251.6 ± 107.2 |
| Glucose (mmol/L) | 129.6 ± 86.9 | 134.2 ± 81.8 | 110.4 ± 58.1 | 115.6 ± 62.7 | 98.0 ± 36.6 | 100.4 ± 37.8 |
| HbA1c (mmol/mol) | 6.5 ± 1.3 | 6.4 ± 1.6 | 6.2 ± 1.5 | 6.1 ± 1.7 | 5.6 ± 1.4 | 5.6 ± 1.4 |
| CRP (mg/L) | 9.8 ± 5.8 | 10.1 ± 4.8 | 11.2 ± 5.2 | 10.3 ± 4.8 | 10.2 ± 5.6 | 9.9 ± 5.8 |
| Ferritin (µg/L) | 222.7 ± 168.0 | 250.1 ± 179.3 | 485.7 ± 167.2 | 347.4 ± 178.2 | 156.0 ± 166.2 | 116.4 ± 110.0 |
| AST (µg/L) | 23.7 ± 17.8 | 22.5 ± 15.1 | 30.8 ± 18.5 | 23.5 ± 15.8 | 24.4 ± 17.3 | 22.2 ± 14.8 |
| ALT (µg/L) | 28.8 ± 19.4 | 27.1 ± 19.9 | 36.9 ± 20.8 | 28.0 ± 19.2 | 28.7 ± 19.0 | 26.7 ± 18.3 |
| ALK (µg/L) | 82.3 ± 60.8 | 85.1 ± 63.9 | 89.5 ± 49.3 | 84.0 ± 60.8 | 115.4 ± 57.4 | 88.4 ± 51.9 |
| GGT (µg/L) | 30.8 ± 27.4 | 28.2 ± 19.1 | 27.2 ± 26.0 | 24.2 ± 18.6 | 24.5 ± 23.5 | 19.7 ± 14.7 |
a Values are expressed as Mean ± SD.
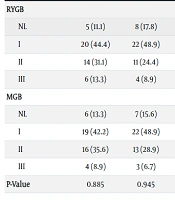
After six months, both groups showed a significant decrease in the grading of fatty liver, with no significant differences between the two groups Table 6.
| Fatty Liver Grading | Time | P-Value | ||||
|---|---|---|---|---|---|---|
| Pre-operation | After 3 Months | After 6 Months | Compare of Pre-operation and After 3 Months | Compare of After 3 Months and After 6 Months | Compare of Pre-operation and After 6 Months | |
| RYGB | 0.025 | 0.020 | 0.001 | |||
| NL | 5 (11.1) | 8 (17.8) | 12 (26.7) | |||
| I | 20 (44.4) | 22 (48.9) | 24 (53.3) | |||
| II | 14 (31.1) | 11 (24.4) | 6 (13.3) | |||
| III | 6 (13.3) | 4 (8.9) | 3 (6.7) | |||
| MGB | 0.043 | 0.015 | 0.003 | |||
| NL | 6 (13.3) | 7 (15.6) | 10 (22.2) | |||
| I | 19 (42.2) | 22 (48.9) | 26 (57.8) | |||
| II | 16 (35.6) | 13 (28.9) | 7 (15.6) | |||
| III | 4 (8.9) | 3 (6.7) | 2 (4.4) | |||
| P-Value | 0.885 | 0.945 | 0.910 | |||
a Values are expressed as (%).
5. Discussion
Laparoscopic SG and RYGB are currently the most popular bariatric procedures. However, in recent years, MGB has been gaining increasing popularity (25, 26). Based on the 2019 International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) Global Register, one-anastomosis gastric bypass (OAGB) is notably more prevalent than RYGB in several countries, including India, Turkey, Russia, and Qatar, among those with over 4000 entries in the registry (27). While MGB may seem appealing due to its shorter operation time, ease of use, and lower rates of morbidity and mortality, RYGB is often considered the gold standard procedure. Another advantage of MGB is the single anastomosis that is clearly visible, reducing the risk of leakage (25, 28). The occurrence of severe ALI and ALF following bariatric surgery is not well-established. However, recent studies suggest that it may affect only a small number of individuals globally each year. Acute liver injury and ALF typically manifest several months after bariatric surgery. To prevent these severe complications, it is recommended that patients at a high risk of developing liver failure after bariatric surgery undergo comprehensive preoperative evaluations. Personalized treatment plans should be also considered for these patients (22, 29).
The RYGB, a longstanding bariatric procedure, has been the gold standard for treating metabolic disorders and morbid obesity for over four decades. However, the adoption of OAGB is on the rise worldwide due to its simplicity and safety. This trend is supported by evidence from randomized trials and long-term data, leading many surgeons to prefer this procedure (9, 30-33).
Our study significantly contributes to the existing literature by comparing the recovery from NAFLD after RYGBP and MGB as two common bariatric procedures. While previous research acknowledges their effectiveness in improving NAFLD and liver function, there is limited comparative analysis for this purpose. Our study also identified risk factors for the decline in liver function postoperatively, thereby filling a crucial knowledge gap. In our study, the majority of participants in both groups were female, which could be attributed to the higher obesity rates among women and societal beauty standards. However, the gender differences between the two groups were not significant. The average age of the patients studied was 38.6 ± 10.4 years. Given the typical age range for bariatric surgeries and the fact that most young people are candidates for these procedures, the average age in this study is justifiable. We controlled for the effects of age and gender by matching the two groups for these variables. It is worth noting that half of the patients were 31 to 45 years old.
When an individual becomes overweight or obese, they may become prone to other chronic diseases, including metabolic syndrome. A person is diagnosed with metabolic syndrome if they meet three or more of the following criteria: A waist circumference of 102 cm (40 inches) or more in males and 88 cm (35 inches) or more in females, triglycerides levels of 150 mg/dL or higher, HDL-C levels less than 40 mg/dL in men and less than 50 mg/dL in women, blood pressure of 130/85 mmHg or higher, and fasting glucose levels greater than 100 mg/dL (34). In this study, certain factors were examined to gain a better understanding of liver function following bariatric surgery. As demonstrated by Lee et al., laparoscopic mini gastric bypass (LMGBP) is a faster and safer method than LRYGBP for addressing metabolic issues caused by morbid obesity and enhancing the quality of life of patients (31).
Following the procedures, we observed that blood pressure remained unaffected in both groups throughout the entire period, despite weight loss being a known method to reduce blood pressure. All procedures that resulted in weight loss were carried out within the two years post-surgery (35).
Postoperatively, both groups showed improvements in HbA1c and fasting glucose levels, with no significant differences between the two after six months. Based on these results, bariatric surgery could be considered a viable alternative to conventional treatments for severe obesity resulting in diabetes (36).
Three months after surgery, there was no significant difference in terms of weight loss and BMI between the two surgical methods. However, six months after surgery, patients who underwent MGB had significantly lower average weight and BMI compared to those who underwent RYGB. In other words, while both MGB and RYGB resulted in similar short-term weight loss (three months), MGB was more effective than RYGB at six months postoperatively. It is worth noting that the sagittal diameter remained consistent in both groups throughout the entire period. This aligns with a report by Chetan Parmar et al., which indicated that OAGB/MGB resulted in greater weight reduction than RYGB in approximately two-year follow-ups (37).
Our study found that neither of the two surgical methods (MGB and RYGB) had a significant impact on the average count of PLT, hemoglobin, and MCV at three and six months postoperatively. In the RYGB method, the serum level of CRP was significantly higher three months after surgery compared to the preoperative levels, and the increase in serum ferritin level at three months post-surgery was significantly higher in the RYGB group than in the MGB group. There was a significant difference between the RYGB and MGB methods in terms of two inflammatory factors, that is, CRP and ferritin. This suggests that the RYGB method is more aggressive, potentially leading to more postoperative complications. A report by Antoniewicz et al., which compared the LSG and RYGB methods over 1 - 12 months, corroborates the findings of our study.
Obesity is a potential cause of conditions, such as non-alcoholic steatohepatitis (NASH) or NAFLD. Bariatric surgery is acknowledged as an effective treatment option for these conditions (38-41).
Just as a certain percentage of NAFLD/NASH patients in the general population will develop cirrhosis and liver failure, some NAFLD/NASH patients who undergo RYGB may also experience these conditions. Currently, there is no comprehensive comparison in the existing literature to substantiate the claim that RYGB reduces the incidence of liver failure in NAFLD/NASH patients, even though this expectation seems logical (42). A biopsy is often considered an invasive procedure with potential risks, such as bleeding and bile leakage. Other methods for screening and monitoring NAFLD, such as biochemical testing, scoring scales, and radiological examinations, have been proposed. However, none of these alternatives have been widely adopted. Consequently, the most commonly performed tests to assess liver disease remain the ALT and AST tests (42). While LFTs often show significant improvement following RYGB, it is important to note that many patients may still exhibit abnormal LFTs years after the surgery (44). Weight loss surgeries can change the levels of AST, ALT, apolipoprotein, and GGT in the parenchymal liver (45). The primary objective of this study was to assess how MGB and RYGB surgeries influenced the progression or regression of liver function in obese patients who underwent bariatric surgery. The MGB procedure did not significantly impact the levels of ALT and AST in patients at three and six months postoperatively. In contrast, the RYGB procedure led to a significant rise in the serum ALT and AST levels three months after surgery, but these levels decreased six months after surgery compared to the three-month follow-up.
In both groups, the serum levels of ALT and AST were not significantly different six months after surgery compared to the preoperative levels. In essence, this study demonstrated that, compared to MGB, RYGB causes a significant increase in ALT and AST enzymes at three months postoperatively, which nearly return to preoperative levels at six months postoperatively. The MGB procedure did not affect the average ALP of patients at three and six months postoperatively. In contrast, the RYGB procedure significantly elevated the blood level of ALP at six months after surgery, compared to its levels before and three months after the procedure. In other words, this research indicated that the RYGB approach resulted in a substantial increase in ALP in the six-month follow-up, a level that remains significantly higher than that observed in the MGB procedure.
Our research revealed that three months after surgery, patients in the MGB group exhibited a significant decrease in the GGT levels compared to those in the RYGBP group. This suggests that GGT could potentially serve as a predictive marker for improved inflammation and fibrosis in NAFLD following weight loss (46). For clarification, this study found that the average GGT levels of patients were not significantly influenced by the RYGB procedure in three- and six-month follow-ups. However, the MGB approach significantly decreased the serum GGT levels six months after surgery, compared to GGT levels before and three months postoperatively. In other words, this research showed that, compared to RYGB, the MGB surgery resulted in a substantial decrease in GGT six months after surgery, a reduction that is still evident in the six-month follow-up. These findings align with those of numerous previous studies.
A study by Moolenaar et al. supports our findings. Their study showed that bariatric procedures, such as jejunoileal bypass (JIB) and biliopancreatic diversion (BPD), which cause significant abnormalities in the gastrointestinal structure, should be avoided as much as possible to minimize the risk of ALI and ALF following these procedures (22). In a 12-month trial conducted by Kalinowski P et al., both the RYGB and SG groups exhibited reductions in aminotransferases, GGT, and LDH. However, the improvement was deemed significant only in the SG group, where the anatomical changes were less pronounced than in the RYGB group and comparable to those in the MGB group (47). Earlier studies have suggested that rapid weight loss following bariatric surgery could potentially have adverse effects on the liver. Additionally, previous research has shown that the omega-loop gastric bypass surgery achieves superior weight loss outcomes compared to RYGB (23, 31, 48, 49). Indeed, the study by Kruschitz et al. presents contrasting findings. They observed that three months post-surgery, the increase in liver transaminases (ALT and AST) was most pronounced in patients who underwent omega-loop gastric bypass, as opposed to those who underwent RYGB. The discrepancy in results could be attributed to several factors, including the number of patients involved in the study or variations in the surgical procedure itself (44).
Our study revealed that MGB leads to more significant weight loss and a greater reduction in BMI compared to RYGB. However, neither RYGB nor MGB had a significant impact on abdominal circumference, systolic blood pressure, diastolic blood pressure, hemoglobin levels, MCV percentage, or platelet count. Interestingly, the RYGB method was found to significantly increase the ALP, AST, ALT, ferritin, and CRP levels when compared to the MGB method. In terms of their impact on fatty liver grading and serum levels of fasting blood sugar and hemoglobin A1C, both RYGB and MGB showed similar effects, and neither was preferred over the other.
5.1. Conclusions
The MGB method can be arguably favored over the RYGB procedure due to the lesser degree of anatomical alteration during the operation. This results in a reduced stimulation of inflammatory factors within the patient’s body, leading to a decrease in the elevation of liver enzymes. This, in turn, expedites the postoperative healing process and minimizes the risk of complications. Furthermore, it has been observed to result in greater weight loss six months after surgery.
In future research, it's essential to explore the long-term outcomes and effectiveness of various bariatric surgeries on liver health, including their impact on liver function test (LFT) enzymes. Additionally, prospective studies should examine their influence on patient survival rates while considering potential confounding factors. Integrating assessments like liver biopsy or FibroScan could offer valuable insights into liver histology and fibrosis post-surgery, enhancing the comprehensiveness of future studies.