1. Background
Hepatitis B virus (HBV) infection remains one of the major infectious agent threats to the human health, it is estimated that this virus infects 400 million of the world’s population and is considered as one of the most common transfusion-transmitted viral infection (1-5). Occult HBV infection (OBI) was defined by an international workshop held in 2008 as the “presence of HBV DNA in liver (with a viral load of < 200 IU/mL or undetectable HBV DNA in the serum) of individuals testing hepatitis B surface antigen negative by currently available assays” (6-8). The prevalence of OBI carriers was shown to be more prominent since the introduction of HBV DNA nucleic acid testing (NAT) in blood transfusion centers across all continents with yield varying from 0.1% to 4.16% (2-5, 9-22).
In 2015, the Lebanese committee of blood transfusion, with the approval of the ministry of health implemented a new criterion for HBV blood safety; any anti-HBc (antibody to hepatitis B virus core antigen) positive blood unit triggered an indefinite deferral of donor. This strategy resulted in permanent donor deferral of 3% - 5 %. So far, many countries started to implement NAT screening in minipools for all their blood donations along with anti-HBc. Considering that Lebanon is a country of low/medium HBV endemicity, the implementation of NAT is not yet considered.
This study aimed to determine the prevalence of occult HBV in blood donors in Beirut, Lebanon with a testing algorithm for HBV markers including HBsAg (Hepatits B surface antigen), anti-HBs (Antibody to hepatitis B virus surface antigen), and DNA in anti-HBc reactive donors. The outcome of this study was used to support whether or not a decision of implementing HBV NAT in Lebanese blood donor settings is justified.
2. Methods
2.1. Sample Collection
This cross-sectional study estimated the prevalence of OBI in blood donor samples collected between August 2013 and March 2015. A total of 7437 serum samples were collected consecutively during a 20 month period in the transfusion center of a major hospital in Beirut, Lebanon. The study was approved by the institutional review board of Makassed general hospital. Donors were asked about their age, gender, nationality, and HBV vaccination status.
2.2. Serological Analysis
All blood donors were tested for anti-HBc using the ARCHITECT Anti-HBc II chemiluminescent microparticle immunoassay (Abbott Diagnostics, Delkenheim, Germany). Architect HBsAg (Abbott Diagnostics), Hepanostika HBsAg Ultra (BioMerieux, France), and MonolisaTM Anti-HBs PLUS (Bio-Rad, Costa Mesa CA, USA) were used to test all anti-HBc positive reactive samples for HBsAg and anti HBs, respectively, according to the manufacturers’ instructions. HBsAg positive samples were tested for Anti-HBe (Antibody to hepatitis B virus e antigen) with the Cobas anti HBe kit (Roche Diagnostics, Mannheim, Germany).
2.3. Detection of HBV DNA
DNA was extracted from 500 μL of plasma using the high pure viral nucleic acid kit and eluted in 50μl of elution buffer, according to the manufacturers’ instructions (Roche Diagnostics, GmbH, Germany). Two assays were used to detect HBV DNA in anti-HBc positive samples: Artus HBV TM PCR kit (sensitivity of ≤ 3.8 IU/mL), targeting the pre-core gene, was used according to the manufacturers’ instructions (Qiagen, Hilden, Germany) and an in-house assay using the Platinum PCR kit (Invitrogen, San Diego, USA) was tested in parallel targeting the S gene of HBV using the following primers: HBV-1 (CAACCTCCAATCACTCACCAAC) and HBV-2 (ATATGATAAAACGCCGC AGACAC). Dilutions of WHO international standard plasma sample (NIBSC, Potters’ Bar, United Kingdom; 97/746) were tested as assay controls. The 25 µL PCR (polymerase chain reaction) reaction included 5 µL of purified nucleic acid, 12.5 µL of 2× reaction mix containing 4 mM MgSO4, 1 µM of forward and reverse primers HBV-1 and HBV-2, 0.2 µM of fluorogenic probe, as previously described (23). The amplification cycle conditions were as follows: a cycle of 95°C for 10 minutes, then 50 cycles of 30 sec at 95°C and 1 minute at 60°C. The in house assay sensitivity is 20 IU/mL (24), however, some samples were quantified below that threshold. Quantification assays were performed on the CFX96 real-time PCR instrument (Bio-Rad, CA, USA).
2.4. Nested PCR for the S Gene
All anti-HBc positive samples were tested by nested PCR, targeting the S gene (494bp) using the Taq DNA Polymerase (Sigma-Aldrich, St. Louis, USA). The outer primers were HBVS1 and HBVS2 and inner primers were HBVS3 and HBVS4 (25, 26). The first and second round amplifications were performed in a total volume of 50 µL, containing 1× reaction buffer, 0.2 mM dNTPs, 1.5 mM MgCl2, 0.4 µM of each primer, 0.5 µL of Taq DNA polymerase, and 10 µL of DNA. The cycle conditions were 95°C for 2 minutes, followed by 38 cycles at 94°C for 20 seconds, 55°C for 30 seconds, and 72°C for 45 seconds. A final 10 minutes elongation step at 72°C was performed. Five μL of the amplified product of the first round was used as a template for the second round PCR using HBVS3 and HBVS4 with similar cycle conditions.
2.5. Statistical Analysis
In this study, the statistical analysis was done using the SPSS software (version 20). Categorical variables were compared by using the Chi-square exact test. P values less than or equal to 0.05 were considered significant.
3. Results
3.1. Demographic Characteristics of Blood Donors
A total of 7437 blood donors were included in this study (Table 1), 25% were voluntary donors and 75% were replacement donors. The age of the donors ranged from 18 to 67 years with a median of 38 years old. The majority of the donors were Lebanese (n = 5608/7437, 75 %) or Syrian (n = 1406/7437, 19%), 22 donors were Palestinian (n = 22/7437, 0.3%) and 5 were from different nationalities including Egyptian, Iraqi, Iranian, Turkish, or American (n = 5/7437, 0.06%). Among the selected blood donors, 96% were males and 4% were females. Only 3 individuals confirmed that they were vaccinated for HBV.
| Variables | Value |
|---|---|
| Duration of study | 20 months |
| Total number of blood donors | 7437 |
| Demographic Data | |
| Age | Range: 18 - 67 (median: 38 years) |
| Nationality | |
| Lebanese | N= 5608 (75) |
| Syrian | N= 1406 (19) |
| Palestinian | N= 22 (0.3) |
| Gender | |
| Female | N= 7139 (96) |
| Male | N= 298 (0.04) |
| Vaccination | N= 3 (0.04) |
| Serological Data | |
| Anti-HBc positive donors | N= 341 (4.6) |
| HBsAg positive in anti-HBc seropositive blood donors | N= 21 (6) |
| Anti-HBs positive in anti-HBc seropositive blood donors | N= 236 (69) |
aValues are expressed as No. (%).
3.2. Serological Characterization of Blood Donors
Anti-HBc was screened during a 20 month period. There were 341 blood donors who were anti-HBc positive (n = 341/7437, 4.6%). Among the anti-HBc positive blood donors, 21 were HBsAg positive (n = 21/341, 6.2%; overall prevalence 0.28%).
There was a significant difference in anti-HBc, HBsAg, and anti-HBs prevalence between Lebanese, Syrian, or Palestinian blood donors (Table 2); 1.6% (n = 3/184), 11.5% (15/130), and 13.6% (n = 3/22) were HBsAg positive, respectively (P value <0.05). Fifteen of the HBsAg positive samples were confirmed anti-HBe positive.
| Nationality | Total number of blood donors | Prevalence (Prevalence %) | ||
|---|---|---|---|---|
| Anti-HBc | HBsAg | Anti-HBs | ||
| Lebanese | 5608 | 184 (3.2) | 3 (0.05) | 148 (2.6) |
| Syrian | 1406 | 130 (9.2) | 15 (1.07) | 93 (6.6) |
| P value | < 0.01 | < 0.01 | < 0.05 | |
| Palestinian | 294 | 22 (7.4) | 3 (1.02) | 13 (4.4) |
| P value | < 0.05 | < 0.05 | > 0.05 | |
The frequency of anti-HBs positive samples among anti-HBc positive/HBsAg negative samples was 80% (n = 256/320). The anti-HBs titer ranged between 10 and 1000 mIU/mL with a median of 200 mIU/mL (< 10 mIU/mL was considered negative).
3.3. Molecular Analysis of Anti-HBc Seropositive Blood Donors
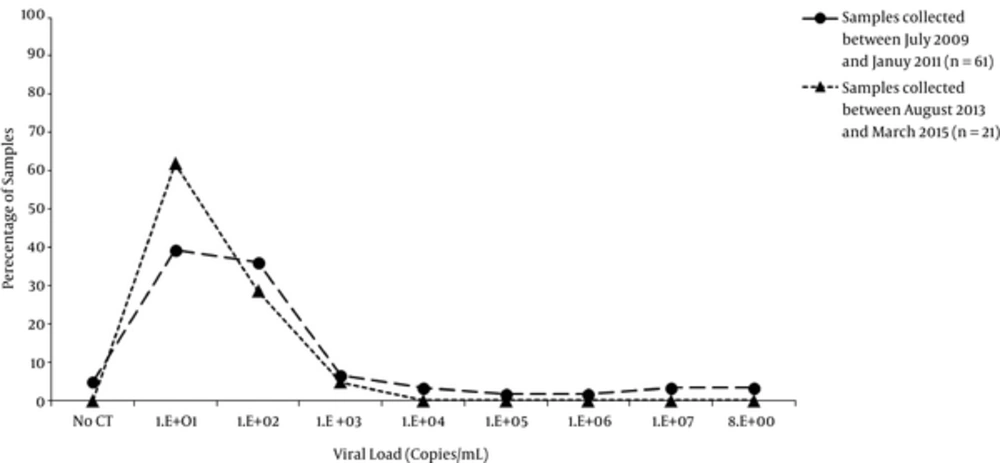
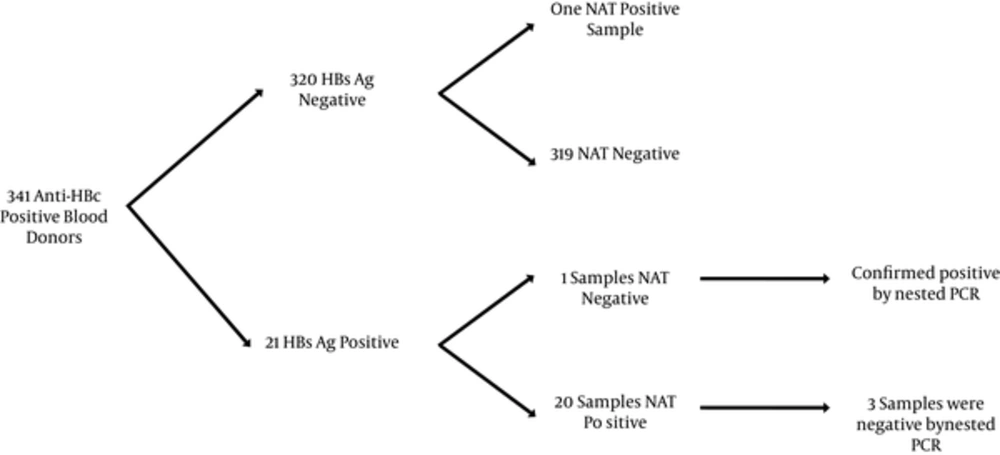
A total of 21 samples were DNA positive; the viral load, quantified by Artus HBV TM PCR kit, ranged from 2 to 1162 IU/mL (median of 9.3 IU/mL) with 95% (n = 20) of the samples below 500IU/mL (Figure 1). One sample had a viral load of 1162 IU/mL, 5 samples between 179 and 453 IU/mL as well as 15 samples between 2 and 48 IU/mL. Fifteen of the 20 HBsAg positive samples had enough volume left to test for HBeAg/anti-HBe and all were anti-HBe positive.
The prevalence of occult HBV (HBsAg negative and NAT positive) in anti-HBc positive samples was 0.3% (1/320). This unique qPCR positive sample was quantified by Artus HBV TM PCR Kit with a viral load of 4 IU/mL. This sample was found in a 49 year old male Syrian donor who was also anti-HBs positive (1047 mIU/mL).
All anti-HBc positive samples were tested by nested PCR. The donor carrying the occult infection was confirmed positive by nested PCR and all samples negative by NAT were also confirmed negative by nested PCR.
Among the HBsAg positive samples, there were some discrepancies between real time PCR and nested PCR assays. One sample was negative for HBV nucleic acid by real-time PCR but positive by nested PCR. In addition, 3 samples were positive for DNA by real-time PCR, however, negative by nested PCR (Figure 2). Two of these samples had a viral load below 10 IU/mL and 1 sample had a viral load of 1162 IU/mL.
4. Discussion
In this study, the objective was to identify OBI among the deferred blood donors. In order to do so, 7437 blood donors were first tested for anti-HBc during a 20 month period. Month by month the prevalence varied between 2.9% to 7.2% with an average of 4.6%. Architect Anti-HBc II assay has a good performance in detecting antibodies to hepatitis B core antigen (27, 28), however discrepancies and low predictive value of anti-HBc assays in countries with low HBV prevalence were previously reported (29-33). When the prevalence was measured based on the nationality, 9.2% (n = 130/1406) of Syrian and 3.2% (184/5608) of Lebanese were anti-HBc positive, respectively. A similar prevalence (3.7%) was observed in Lebanese blood donors in 2005 - 2006 by El-Zaatari et al. (34). HBV infection in blood donors is often seen in non-Lebanese citizens, as 11.8% (n = 18/152) and 6.2% (n = 106/1700) of anti-HBc positive samples were HBsAg and anti-HBs positive, respectively, as compared to Lebanese with 1.6% (n = 3/184) and 2.6% (n = 148/5608) (Table 2). All HBsAg positive samples tested were confirmed positive for anti-HBe suggesting that these blood donors were HBV carriers with relatively low viral load as confirmed by DNA quantification (Figure 1). The majority of these HBsAg positive healthy donors are in the low or non-replicative phase of chronic hepatitis B infection and do not require anti-viral therapy.
In a previous study conducted between July 2009 and January 2011, 61 HBsAg positive donors were tested for NAT (35) and when compared to the current study, 21 HBsAg were positive. Both of studies have a comparable distribution of HBV viral load at 2 different periods of time, with a predominance of a low viral load in anti-HBe positive, asymptomatic, blood donors (Figure 1). Therefore implementing a highly sensitive assay for HBV DNA screening in Lebanese blood donors, should be considered to avoid any false negative results. Previous studies have shown that various NAT commercial assays differ in their sensitivity (36-38), this could be associated with various factors such as: lower input of extracted samples, sample pooling, and mutations in the target genes.
Chaar et al. previously sequenced 42 HBV full genome strains isolated from Lebanon and demonstrated that genotype D strains were the only circulating viruses; in addition various mutations were found in the S and core genes, those were associated with escape detection or disruption of protein synthesis (35, 39, 40)
In this study, the prevalence of OBI in healthy anti-HBc seropositive blood donors was 0.3% (n = 1/341), only 1 Syrian donor was an OBI carrier with a high anti HBs level > 1000 mIU/mL. Molecular analysis for the detection of any mutation associated with the occult strain was not studied due to plasma volume limitation. A previous Lebanese study conducted in 2007 reported an OBI prevalence of 5.4% (n = 11/203), which is higher than what was observed in the current study. However, El-Zaatari et al. detected the circulating viral DNA by nested PCR but failed to detect it by Amplicor HBV monitor test that had a lower sensitivity (400 genome copies/mL) than the current available commercial assays (34).
A variable prevalence of OBI was reported in few countries of the MENA region (Table 3) such as in Syria (8%), Iran (0.4%), Egypt (11.5% to 17.2%), and Saudi Arabia (0.4%) (2, 34, 41-44). The prevalence rates previously stated may differ according to the sensitivity of the NAT assay used in each country. Lebanon has over 1,000,000 Syrian refugees since the start of the Syrian civil war, many epidemics of communicable diseases were reported in this displaced population (45). Among the Lebanese population, the prevalence of HBV is low; only 3 donors were HBsAg positive. The highest prevalence was seen in Syrian as well as Palestinian blood donors (Table 2).
There are many challenges that investigators face but one of them is the follow-up of patients with OBI, which can provide additional information regarding the patients’ status. In the current study, we recalled the donor who was positive by NAT and retested him for all HBV serological markers and for the presence of nucleic acid. Three years later, the donor was still positive for anti-HBc and anti-HBs, however he was confirmed negative for HBV nucleic acid using the NAT and nested PCR. Candotti et al. demonstrated, in a look-back study from 25 blood donors who donated 2 to 5 donations over a 20 month period, that the viral load may fluctuate over time and can fail nucleic acid detection (12). Therefore negative NAT does not necessarily indicate that the donor is free of circulating HBV, but possibly that the virus has been replicating at very low level in the liver with occasional release of undetectable viral particles in the circulation. Analysis of HBV DNA in liver biopsy material would be useful to confirm a true OBI.
| Country | Period of Sample Collection | Sample Size | Prevalence, % | Reference | |||
|---|---|---|---|---|---|---|---|
| Anti-HBc positive samples/ Total blood donation | HBsAg / Total blood donation | OBI/ Anti-HBc + | OBI/ Total blood donation | ||||
| Egypt | NS | 3167 | 16.6 ( n= 525) | NS | 9.9 (n = 52) | 1.6 | (2) |
| Egypt | 1 month (2005) | 712b | 10.9 (n = 78) | 1.2 | 11.5 (n = 9) | 1.26 | (34) |
| Iran | 10 months (2008 - 2009) | 5000 | 9.9 (n = 499) | NS | 0.4 (n = 2) | 0.04 | (41) |
| Saudi Arabia | 4 months (2005 and 2007) | 600c | 11.5 (n = 69) | 0.3 | 4.3 (n = 3) | 0.5 | (42) |
| Syria | 6 months (2011) | 3896 | 12 (n = 468) | 1.7 | 1 (n = 5) | 0.1 | (44) |
| Lebanon | 12 months (2005 - 2006) | 5511 | 11 (n = 608) | 0.9 | 4.3 (n = 11) | 0.2 | (32) |
| Lebanon | 20 months (2013 - 2015) | 7437 | 4.6 (n = 342) | 0.3 | 0.3 (n = 1) | 0.01 | current study |
Abbreviation: NS, The Data Were Not Specified in the Article.
aA total of 253 Anti-HBc positive samples were tested for HBV DNA (Anti-HBc alone’ (n = 203), Anti-HBc./anti-HBs. (n = 50). A total of 355 anti-HBc/ Anti-HBs positive samples were not tested for HBV DNA.
bIn this study, authors have collected healthy blood donor volunteers, who were referred to National Blood Transfusion Center and mobile blood collection vehicles during September 2005. Samples collected were then tested for HBV NAT.
cThe collection started in February 2005, and the second period started in April, 2007. Both periods continued for 2 months.
In this study, we performed both real-time and nested PCR for HBsAg positive and occult samples. Discrepant results were found for some HBsAg positive samples; 3 samples were positive by real time PCR and negative by nested PCR, while 1 sample was negative by real time PCR and positive by nested PCR assays. Failure of detecting HBV DNA using NAT assays was previously reported (25). Inconsistency in the results can be observed when there is low level of circulating HBV or upon the presence of mutations in NAT assay targeted region.
This study has few limitations; the prevalence of occult HBV infection was studied from 1 blood donor center, which does not represent the prevalence in a country. A multicenter study from different Lebanese regions would better represent the country prevalence. In addition, this study did not focus on the prevalence of OBI in the Lebanese versus non-Lebanese communities. A larger sample size from both Lebanese versus Syrians refugees would assure the future threat of HBV on the Lebanese community.
In conclusion, our study has demonstrated that HBV DNA is present in a small percentage of HBsAg-negative, anti-HBc-reactive units. In the current study, 4.6% of donors were anti HBc positive while only 0.3% of them were viremic. Introducing HBV NAT is more favorable in high-endemic areas, where OBI prevalence is high, compared to those with low to medium endemicity of HBV infection. The disadvantage of not implementing NAT testing is the risk of transfusion blood unit from a donor in the window period and in seronegative (anti HBc negative) OBI donors (46). A better strategy of screening should be reconsidered to decrease the number of blood deferrals in Lebanon such as serological testing of both anti HBc followed by anti-HBs or implementation of NAT along with anti-HBc.