1. Context
Tattooing has arisen from ancient times in many parts of the world (1, 2). In the United States, 24% of people aged 18 to 50 years have had at least one tattoo (3). According to a study conducted in Canada, 8% of high school students have had at least one tattoo on their bodies. In addition, 21% of people who do not have any tattoo on their bodies would like to have tattoos (4). In order to mark a tattoo, pigments are injected into the dermal layer of skin by puncturing the skin 80 to 150 times a minute. Therefore, it is obvious that tattooing tools are in contact with blood and body liquids and if the tattoo needles are reused for more than one person without performing proper sterilization process and hygiene techniques, the blood-borne diseases may be transmitted (5). Moreover, tattoo colors are usually kept in dirty dishes that are a good place for viral and bacterial pathogens and increase the risk of blood-borne diseases (5). Several studies reported a possible association between anti-HCV positivity and tattooing (6-12). The prevalence of HCV infection has been estimated about 3% in the world (13). There are approximately 170 million people with chronic hepatitis C virus (HCV) infection (14, 15). HCV infection is a major cause of liver cirrhosis, hepatocellular carcinoma (HCC), and end-stage liver disease (16, 17). Thus, HCV infection is a basic public health problem all around the world (18-20). Recently, drug injection is the most important risk factor for transmission of HCV infection. It is confirmed to be the cause of 60% of new HCV transmission cases every year due to sharing needle and drug use equipment (21, 22). Moreover, there is reliable evidence on the association between drug use by injection and hepatitis C; however, tattooing as a risk factor of HCV infection has remained a controversy (12, 23-26). Several articles have discussed tattooing as a rout of HCV transmission; however, other studies have not reported any significant association (23, 25-41). Early studies evaluating the relationship between the habit of tattooing and hepatitis C in the United States had recruited a small sample size (less than 100 in case-control studies and less than 2000 in cross-sectional studies). Hence, they were not able to report adjusted odds ratios (42). Moreover, studies that have shown a positive correlation between tattooing and hepatitis C before 1992 were not able to control the effects of known risk factors for hepatitis C including blood transfusion and injecting drug use. History of transfusion before 1992 and IDUs history are the main risk factors for HCV infection and other risks are important as additive risks such as tattooing. Therefore, there are limitations in the interpretation of results (43). However, in a study in 2012, it was pointed out that the significance of these routs of transmission was clear in high-risk groups, but not clear enough in general population (42). One of these high-risk groups is prisoners, because prisoners have strong interest to tattooing. The overall prevalence of hepatitis C among prisoners is estimated to be 25.2% to 37.4% (44-48). About half of the prisoners may be unaware of their serological status and they probably do not have adequate knowledge on their health (49-53). Statistics show that it is a common habit to reuse tattoo needles and 45% of prisoners share the needles with others. The number of new cases of hepatitis C that occur due to tattooing is important for clinical and health care professionals. Nonetheless, there is a contrast between the results of epidemiological studies on the relationship between hepatitis C and tattooing. Consequently, the aim of the present study was to conduct a systematic review of these studies to determine the risk of hepatitis C among people who have tattoos.
2. Methods
2.1. Search Strategy
We searched several international databases including Medline, Web of Science, Scopus, Google scholar, SID, Magiran, Iranmedex, EMBASE, CINAHL, and PubMed from 1996 to May 2017. There was no limitation in our search strategy in terms of specific sub-population, languages, or time interval. The search included blood donors, prisoner, IDUs, Non-IDUs, homeless, and sex workers. The initial search strategy was generated using MeSH subject headings “hepatitis” and “tattoo” in MEDLINE. Related keywords and broad subject headings such as ‘hepatitis’ ‘hepatitis C’ and ‘tattooing’ were included later in the search strategy. In order to increase the sensitivity of the search and select a larger number of related studies, we screened the reference section of the retrieved studies and hand-searched the relevant review studies as well as books, abstracts, and key journals relating to hematology, gastroenterology, and hepatitis. EndNote X7, as citation manager software, was used to manage and screen papers from several online databases.
2.2. Study Selection
We included all observational studies (cohort, case-control, cross-sectional) that assessed the association between tattooing and risk of hepatitis C infection in various study populations if they met the eligibility criteria. We used the following three eligibility criteria in the selection of relevant studies: (1) hepatitis C as either primary or secondary outcome, 2) tattooing as either primary or secondary exposure and, 3) reports on relative risks or odds ratios and their 95% confidence intervals (95% CI) or those providing sufficient data (e.g. cross tabulation of hepatitis C and tattooing from chi-square test or odds ratio and corresponding standard error (SE)) to compute these parameters. Two of the co-authors (KM and MA) independently reviewed the titles and abstracts of articles identified through our search strategy for decreasing the bias and excluded articles that did not meet the eligibility criteria. Articles included by either of the two reviewers were assessed for full text review. At the full text level, two reviewers (KM and MA) screened studies independently and included studies that provided relevant data. Citations with disagreement went through reconciliation proofs between the two reviewers, and a third coauthor (MK) provided input as needed.
2.3. Quality Assessment
We used the strengthening the reporting of observational studies in epidemiology (STROBE) checklist to assess the quality of retrieved studies (49, 50). It includes 22 questions that cover different methodological aspects. The highest level of STROBE score was considered 22. If a manuscript obtained lower than 40% of the highest level of STROBE score, it was considered as low quality, 40-70% as middle quality, and more than 70% as high quality. All studies with middle and high quality were included in the main analysis.
2.4. Data Extraction
Two of the co-authors (KM and MA) independently extracted data from the included studies using structured sheets in Microsoft Excel®. Afterwards, they discussed and checked the disagreements with the third coauthor (MA) as indicated. We extracted data considering: (1) authors , (2) publication year, (3) country of study, (4) study design, (5) study population type, (6) age characteristics, (7) sample size, (8) gender distribution and, (9) odds ratio (OR) and 95% CI.
2.5. Statistical Analysis
We used a random-effect model with invers variance weighing method to compute the odds ratios and 95% CI for tattooing and the risk of hepatitis C infection. The Cochran’s Q test and the I2 statistic (51) were used to assess the heterogeneity between studies. We used the Begg’s test and Egger’s test (53) and visually checked the funnel plot (52) to evaluate the possibility of publication bias. The sensitivity analysis was used to assess the effect of each study on the pooled odds ratio estimation and the pooled odds ratio was calculated after excluding every study. The STATA software version 11.0 was used for all computations (StataCorp, College Station, TX).
3. Results
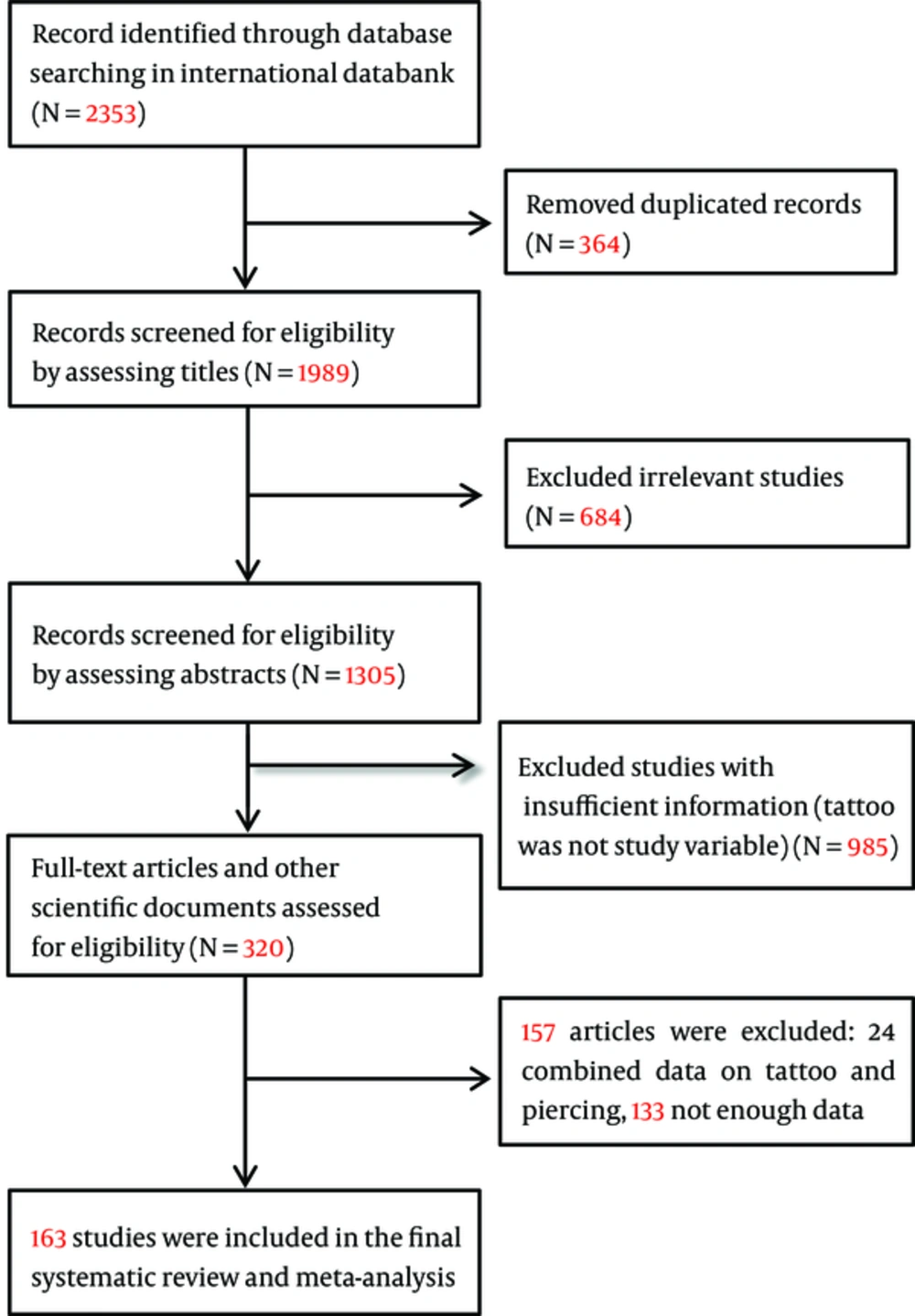
In the first step of search in the electronic databases, we identified 2033 publications relating to risk factors of hepatitis C. In the final step, after removing the duplicates, reviewing the titles, abstracts, and full texts considering the inclusion and exclusion criteria, a total number of 162 studies from 39 countries were selected to enter meta-analysis. Figure 1 shows the details related to step-by-step inclusion and exclusion of the studies. The weighted kappa for eligibility decisions based on the agreement between two reviewers was substantial (Weighted kappa = 0.8, P < 0.001). Characteristics of all the studies included in the systematic review and meta-analysis are shown in Supplement 1. The total sample size of 162 studies (100 cross-sectional, 44 case-control, and 18 cohort studies) that reported an association between tattooing and the risk of transmission of hepatitis C was 327,614 subjects.
3.1. Publication Bias, Pooled Odds Ratio/Relative Risk, and Subgroup Analysis
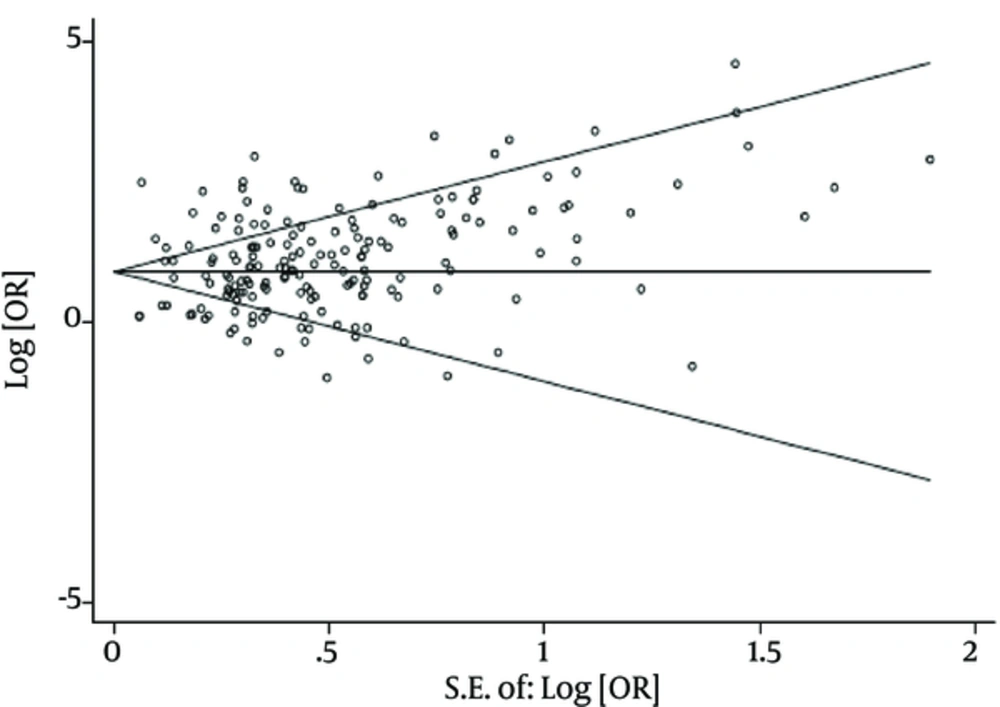
There was no evidence of publication bias based on the results from Egger’s test (P = 0.10) and Begg’s test (P = 0.17). The results of Chi-square test and I2 statistics showed substantial heterogeneity among the studies that reported tattooing as a risk factor of acquiring HCV infection (Q = 2364.65, P value < 0.001, and I2 = 92.6%). Consequently, a random effect model was used to analyze data in this study.
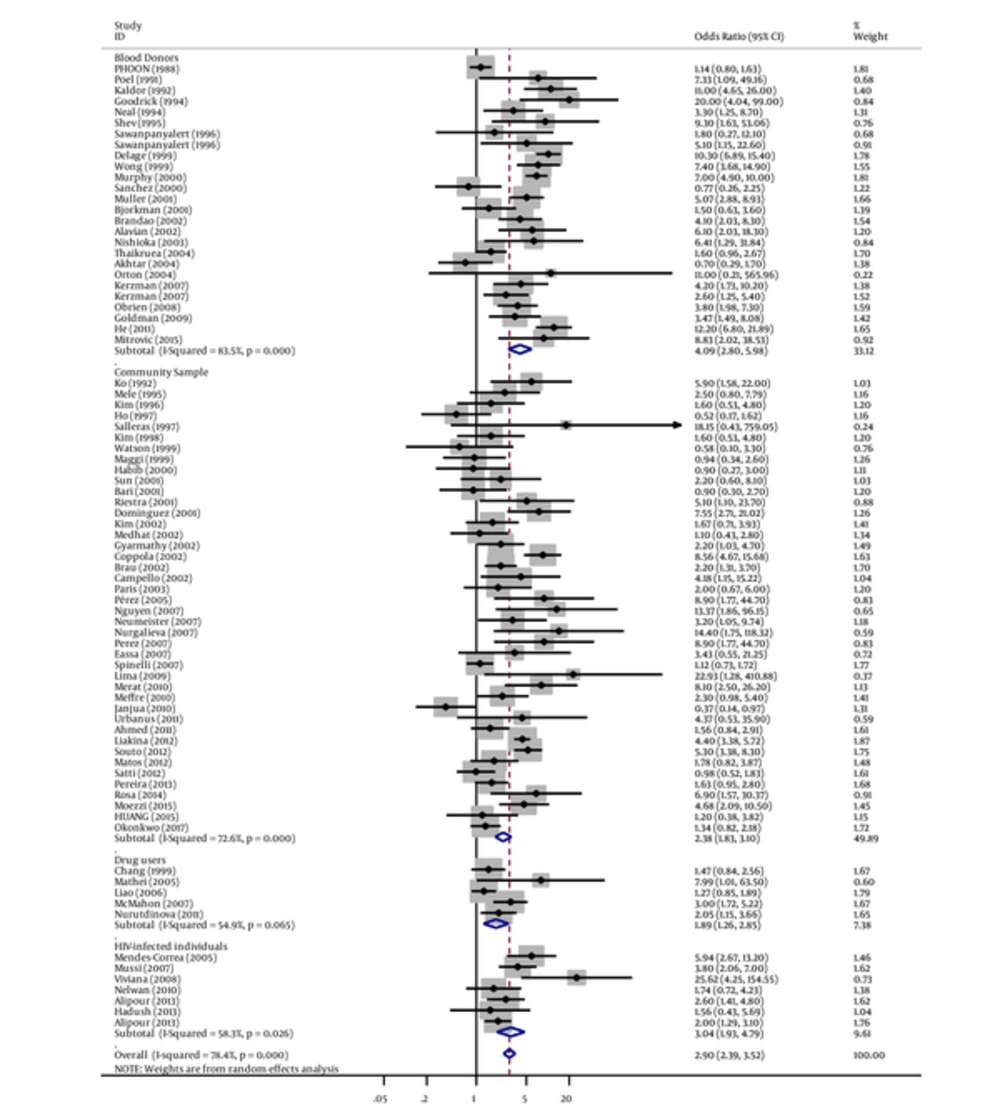
When all studies were combined in the meta-analysis, by applying a random effect model, we found a strong significant association between tattooing and transmission risk of hepatitis C infection (pooled OR = 2.79, 95% CI: 2.46 - 3.18).
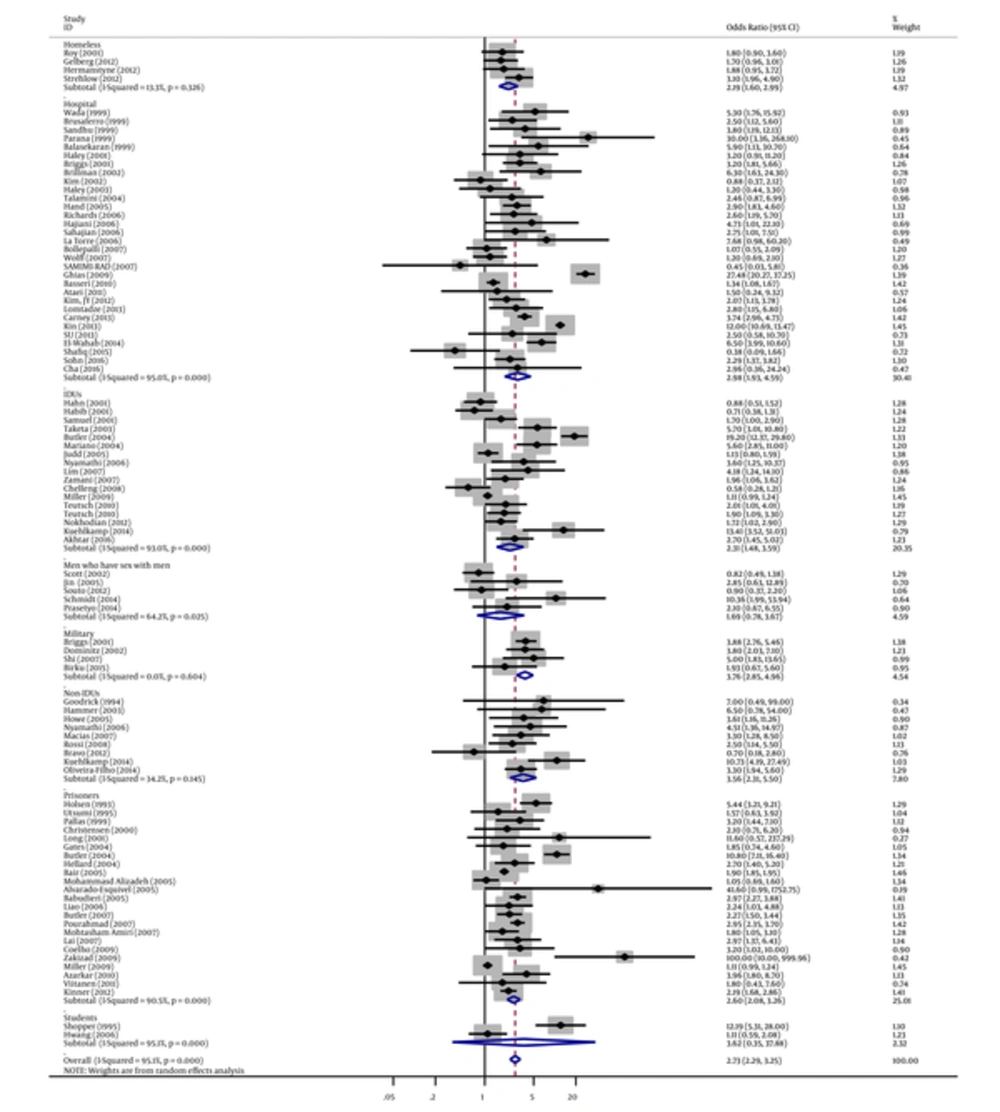
Similarly, in subgroup analyses, we found a strong association between tattooing and risk of hepatitis C for samples derived from blood donors groups (OR = 4.09, 95% CI: 2.80 - 5.98; I2 = 83.5%), followed by military samples (OR = 3.76, 95% CI: 2.85 - 4.96; I2 = 0%), student samples (OR = 3.62, 95% CI: 0.35 - 37.88; I2 = 95.1%), non-IDUs (OR = 3.56, 95% CI: 2.31 - 5.50; I2 = 34.2%), HIV-infected individual samples (OR = 3.04, 95% CI: 1.93 - 4.79; I2 = 58.3%), hospital samples (OR = 2.98, 95% CI: 1.93 - 4.59; I2 = 95%), prisoners (OR=2.60, 95% CI: 2.08 - 3.26; I2 = 90.5%), community samples (OR = 2.37, 95% CI: 1.82 - 3.09; I2 = 72.6%), IDUs (OR=2.31, 95% CI: 1.48 - 3.59; I2 = 93%), homeless samples (OR = 2.19, 95% CI: 1.60 - 2.99; I2 = 13.3%), drug user samples (OR = 1.89, 95% CI: 1.26 - 2.85; I2 = 54.9%), and men who have sex with men (OR = 1.69, 95% CI: 0.78 - 3.67; I2 = 64.2%) (Figures 2 - 4 and Table 1.
| Number of OR/RR Included | Subgroups | Pooled OR (Random Effect) | 95% CI | I-Squared, %a | |
|---|---|---|---|---|---|
| Stydy design | |||||
| 49 | Case-control studies | 3.59 | 2.65 - 4.87 | 91.8 | |
| 107 | Cross-sectional studies | 2.66 | 2.32 - 3.04 | 83.6 | |
| 20 | Cohort studies | 1.64 | 1.35 - 2.01 | 70.8 | |
| Gender | |||||
| 24 | Male | 2.13 | 1.64 - 2.77 | 69.1 | |
| 3 | Female | 5.17 | 0.89 - 30 | 46 | |
| 148 | Total (male and female) | 2.92 | 2.53 - 3.37 | 93.6 | |
| Country | |||||
| Australia | 3 | Group 1b | 4.68 | 1.37 - 15.99 | 77.7 |
| 14 | Group2c | 3.01 | 1.96- 4.63 | 95.7 | |
| Argentina | 2 | Group2 | 6.91 | 0.72 - 66.45 | 81.5 |
| Brazil | 7 | Group 1 | 3.55 | 2.05 - 6.15 | 74.1 |
| 8 | Group2 | 4.13 | 1.99 - 8.59 | 81 | |
| Canada | 4 | Group 1 | 3.51 | 1.11 - 11.11 | 94.5 |
| 2 | Group2 | 2.25 | 1.15 - 4.39 | 14.8 | |
| Egypt | 3 | Group 1 | 1.21 | 0.61 - 2.40 | 0 |
| 1 | Not enough studies (Group2) | - | - | - | |
| UK | 2 | Group 1 | 7.23 | 1.25 - 41.63 | 72 |
| 2 | Group2 | 1.72 | 0.38 - 7.73 | 48.15 | |
| Iran | 3 | Group 1 | 5.72 | 3.24 - 10.10 | 0 |
| 12 | Group2 | 2.21 | 1.59 - 3.8 | 69 | |
| Italy | 4 | Group 1 | 3.14 | 1.08 - 9.17 | 79.2 |
| 5 | Group2 | 3.20 | 2.47 - 4.16 | 5.3 | |
| Korea | 3 | Group 1 | 1.63 | 0.92 - 2.90 | 0 |
| 4 | Group2 | 1.86 | 1.23 - 2.82 | 19 | |
| Pakistan | 5 | Group 1 | 0.89 | 0.56 - 1.40 | 39.2 |
| 3 | Group2 | 3.36 | 0.38 - 29.73 | 97.1 | |
| Spain | 3 | Group 1 | 7.03 | 3.06 - 16.13 | 0 |
| 3 | Group2 | 2.28 | 1.01 - 5.17 | 49.2 | |
| Taiwan | 5 | Group 1 | 1.83 | 0.44 - 7.58 | 74.2 |
| 3 | Group2 | 2.01 | 1.29 - 3.14 | 56.7 | |
| Thailand | 4 | Group 1 | 1.84 | 1.19 - 2.83 | 0 |
| 1 | Not enough studies (Group2) | - | - | - | |
| USA | 9 | Group 1 | 4.02 | 2.14 - 7.54 | 81 |
| 26 | Group2 | 2.60 | 1.77 - 3.81 | 97.6 | |
Results of Subgroup Analysis for Assessing the Association Between Tattooing and Hepatitis C
3.2. Meta Regression Analysis
Meta-regression was used to investigate the effects of suspected variables such as year of study, sample size, study design, and study population in heterogeneity (Table 2). In univariable meta-regression model, there was a significant association between the study design and pooled odds ratio (β = 0.45, P = 0.02). In case-control and cross-sectional studies, the pooled odds ratio was 0.45 times higher than that of cohort studies as reference. Nevertheless, there was no significant association between sample size (β = -0.13, P = 0.35), year of study (β = -0.007, P = 0.49), and study population (β = -0.11, P = 0.38) and pooled odds ratio. In multivariable meta-regression model, the study design was significantly associated with pooled effect size; therefore, the case-control and cross-sectional studies had higher odds ratios in comparison with the cohort studies (β = 0.43, P = 0.03) (Table 2).
Meta-Regression Analysis for Assessing the Effect of Suspected Variables on the Strength of Association
3.3. Sensitivity Analysis
In sensitivity analysis, to assess the effect of each study on the strength of association between tattooing and hepatitis C, the pooled odds ratio was calculated after excluding each study from the meta-analysis. After excluding every study from the analysis, we found no significant difference between the pre-sensitivity pooled odds ratio (OR = 2.79, 95% CI: 2.46 - 3.18) and post-sensitivity pooled odds ratio. The lower and higher pooled odds ratios in the sensitivity analysis were 2.72 (95% CI: 2.44 - 3.04) after omitting the Kin et al. study (W70 in appendix 1) and 2.83 (95% CI: 2.48 - 3.22) after omitting the Janjua et al. (w61 in appendix 1) study. We also repeated the sensitivity analysis for each study population subgroups. We found no significant difference between the pre and post sensitivity pooled odds ratios after excluding each study from the subgroup analysis in blood donors subgroup (OR = 4.09, 95% CI: 2.80 - 5.98), community samples (OR = 2.37, 95% CI: 1.83 - 3.09), drug users (OR = 1.89, 95% CI 1.26 - 2.84), HIV-infected individuals (OR = 3.03, 95% CI: 1.92 - 4.78), homeless individuals (OR = 3.75, 95% CI: 2.84 - 4.95), hospital samples (OR= 2.97, 95% CI: 1.92 - 4.58), IDUs (OR = 2.30, 95% CI: 1.48 - 3.59), men who have sex with men (OR = 1.69, 95% CI:0.78 - 3.67), military forces (OR= 3.75, 95% CI: 2.84 - 4.95), Non-IDUs (OR = 3.56, 95% CI: 2.30 - 5.49), and prisoners (OR = 2.60, 95% CI: 2.07 - 3.25) (Table 3).
| Subgroup | Pre-Sensitivity Analysis | Post-Sensitivity Analysis | |||||
|---|---|---|---|---|---|---|---|
| No. of OR/RR Included | Pooled OR (Random Effect) | 95% CI | Upper and Lower of EFa | Pooled OR (Random Effect) | 95% CI | Excluded Studies | |
| Blood donors | 26 | 4.09 | 2.80 - 5.98 | Upper | 4.41 | 3.03 - 6.41 | Akhtar |
| Lower | 3.86 | 2.64 - 5.66 | He | ||||
| Community sample | 43 | 2.37 | 1.82 - 3.09 | Upper | 2.48 | 1.92 - 3.21 | Janjua |
| Lower | 2.26 | 1.74 - 2.93 | Coppola | ||||
| Drug users | 5 | 1.89 | 1.26 - 2.84 | Upper | 2.20 | 1.43 - 3.38 | Liao |
| Lower | 1.58 | 1.10 - 2.28 | McMAhon | ||||
| HIV-infected individuals | 7 | 3.03 | 1.92 - 4.78 | Upper | 3.42 | 2.00 - 5.84 | Alipour |
| Lower | 2.67 | 1.69 - 4.21 | Mendes-correa | ||||
| Homeless individuals | 4 | 3.75 | 2.84 - 4.95 | Upper | 3.94 | 2.95 - 5.25 | Birku |
| Lower | 3.53 | 2.19 - 5.67 | Briggs | ||||
| Hospital sample | 31 | 2.97 | 1.92 - 4.58 | Upper | 3.15 | 2.03 - 4.87 | Shafiq |
| Lower | 2.71 | 1.77 - 4.14 | Ghias | ||||
| IDUs | 17 | 2.30 | 1.48 - 3.59 | Upper | 2.51 | 1.58 - 3.97 | Chelleng |
| Lower | 1.89 | 1.39 - 2.58 | Butler | ||||
| men who have sex with men | 5 | 1.69 | 0.78- 3.67 | Upper | 2.32 | 0.88 - 6.06 | Scott |
| Lower | 1.12 | 0.66 - 1.89 | Schmidt | ||||
| Non-IDUs | 9 | 3.56 | 2.30- 5.49 | Upper | 3.85 | 2.77 - 5.34 | Bravo |
| Lower | 3.04 | 2.16 - 4.27 | Kuehlkamp | ||||
| Prisoners | 23 | 2.60 | 2.07- 3.25 | Upper | 2.78 | 2.19 - 3.53 | Miller |
| Lower | |||||||
| Military forces | 4 | 3.75 | 2.84 - 4.95 | Upper | 3.94 | 2.95 - 5.25 | Birku |
| Lower | 3.53 | 2.19 - 5.67 | Briggs | ||||
Results of Sensitivity Analysis to Assess the Effects of Every Study on Pooled Odds Ratio
4. Discussion
Since the publication of the previous meta-analysis on tattooing and risk of HCV infection (5), 30 case-control, 8 cohort, and 45 cross-sectional studies have been published. The pooled estimation was comparable with that of previous ones for 36 published studies after 2010 together with studies that were not included in previous meta-analysis. Thus, recent studies confirm the evidence indicating that there is a strong association between tattooing and increased risk of hepatitis C infection. The strength of the present review is owing to its multinational nature of the study participants, large number of studies with different designs and considering various study populations and subgroups. Recent studies enriched the current meta-analysis by providing more evidence on tattooing and its strong association with HCV infection. This strong association remained in all subgroups of the study populations such as community samples, blood donors, prisoners, etc. Some supportive evidence exists about the casual association between tattooing and HCV. First, in some studies, a strong association was reported between HCV infection and the number of tattooing experiences as well as the size of body surface covered by tattoos (54-57). Second, our results are consistent with those of other studies that reported the association between tattooing and other infections such as HIV (58), tetanus (58), Methicillin Resistant Staphylococcus Aurous (59), and leprosy (60) as well as with the findings of a systematic review and meta-analysis of Hepatitis B (59). Finally, the strong association remained in all study populations such as community samples and high-risk populations.
Our results showed the strongest association among high-risk populations such as HIV-infected individuals, prisoners, homeless individuals, IDUs, and drug users. These significant associations may be due to some risky behaviors related to tattooing practice such as needle sharing, reusing tattoo equipment, use of unsterile tattoo equipment, and tattooing in non-professional parlors. The risk of HCV infection due to tattooing may depend on the prevalence of this disease in a target population. Our results indicated that the OR/RR for tattooing among prisoners was higher than that of general populations; however, it was lower than the values of blood donors, military samples, student samples, non-IDUs, and HIV-infected individuals. In previous meta-analyses, the risk of tattooing in prisoners was reported to be lower than the risk of community samples (5). This might be due to a number of reasons including a more detailed subgrouping in our study that separated students and military forces from the general population and differences in the study designs and the sampling methods of the included studies. Another possible reason may be due to that, this study took into consideration new evidence from 2010 publications. Tattooing in the prisoners subgroup is a major concern because of the high prevalence of HCV, HBV, and other blood born infections among this population. In some countries, the prevalence of HCV infection was reported to be 20 times higher among prisoners than the general population (61). Previous studies from Australia, the United States, and Europe indicated that the prevalence of hepatitis C infection in prisons ranges from 8% to 57% (62-65). High risk behaviors as common habits including needle sharing and reusing tattoo needles and equipment were reported 45% among prisoners (45, 66). However, a few prisons worldwide provide sterile needles and syringes for inmates (67-69), which is a measure unlikely to be adopted by most prisons and many countries. The results of this study show the need for establishing prevention programs in prisons to provide safer tattooing practices for prisoners. Moreover, similar interventional and educational programs may decrease the risk of other blood-borne infections among the inmates.
According to the WHO program for combating hepatitis B and C and their elimination by 2030, prevention programs should focus on risk factors and prevent new cases (70). In the general population and other subgroups, some interventions need to be applied to reduce the transmission of hepatitis C infection among people who tattoo. In developed countries, professional tattooists usually use infection control measures and hygienic precautions. Given that the risks for HCV and other blood borne infections are often similar, it is essential to formulate guidelines to emphasize the importance of appropriate infection control measures and develop educational programs for owners of tattoo parlors and tattoo artists, particularly in developing countries. As majority of tattoo recipients are young adults, it is necessary that the education programs focus on this age group to improve their awareness of tattoo-related risks.
Several strategies can be developed to prevent hepatitis C infection among tattoo recipients. Educational programs for infection control standard precautions should be implemented for tattoo recipients, tattoo artists, and tattoo parlor owners. These standards include proper use of autoclaves, single-use sterile tattoo needles, monitoring sterilization process, and appropriate function of disinfectants. Regular monitoring and supervision by health care centers may improve adherence to the standards in tattoo parlors. Records of tattoo recipients should be kept by tattoo parlors and they should report any side effects relating to tattooing to local health centers (71). Finally, it is suggested that clinicians consider screening for hepatitis C and other blood-born viral infections among those who have a history of receiving tattoos as a high-risk population. A limitation of this study is that, because of the observational nature of the studies included in the review, recall bias may affect the results due to lower validity of data gathered in these studies. In addition, information on the history of tattooing may not reflect the current population risk of hepatitis C infection. Further studies are needed to determine the current status of tattooing and risk of hepatitis C in order to gain a comprehensive understanding of the association between tattooing and hepatitis C infection.