1. Context
Thrombocyte levels decline in advanced liver disease due to various processes (1, 2). The primary mechanism behind thrombocytopenia is the sequestration of platelets in the spleen due to portal hypertension. Reduced platelet production also contributes to this condition, with hepatocellular dysfunction resulting in lower levels of thrombopoietin (TPO). Moreover, untreated Heoatitis C virus, infections, alcohol use, nutritional deficiencies, and medications can lead to bone marrow suppression (3).
Current guidelines provide recommendations for managing periprocedural thrombocytopenia. Nevertheless, the majority of clinicians opt for an individualized approach, tailoring management based on each patient's specific condition. This approach integrates various variables to determine whether thrombocyte levels should be increased before procedures or if it's safe to proceed with operations. Thrombocyte transfusions are administered to patients without cirrhosis, typically raising platelet levels by 30,000/μL within 20-30 minutes and by 10 minutes post-transfusion (4). However, this increase diminishes over subsequent days. In patients with cirrhosis, the rise in platelet count after transfusion is only around 12,000/μL (3).
Thrombocyte transfusion, while beneficial in increasing platelet levels, is not without its drawbacks. Limitations include the potential for transfusion reactions, hypersensitivity reactions specifically related to thrombocyte transfusions, transfusion-related acute lung injury, infections, and hemolysis (4). Additionally, a long-term risk associated with repeated thrombocyte transfusions is the development of antiplatelet antibodies, known as alloimmunization. This risk escalates with each subsequent transfusion received by the individual. The refractoriness of thrombocytopenia following transfusion can be attributed to this process (3).
Discoveries regarding thrombopoietin have revolutionized the management of thrombocytopenia, offering a novel approach. Thrombopoietin binds to megakaryocytes and activates TPO receptors, thereby regulating thrombocyte production (5). The efficacy of thrombopoietin-receptor agonists (TPO-RAs) in increasing platelet counts among patients with severe thrombocytopenia scheduled for elective surgeries has been extensively studied (6-20). These agents have proven to be effective in overcoming the limitations of transfusion, serving as an alternative to thrombocyte transfusion. Various investigations, including randomized controlled trials (RCTs), have been conducted to evaluate their effectiveness. Furthermore, the US Food and Drug Administration (FDA) has approved thrombopoietin-receptor agonists for the treatment of cirrhosis and severe thrombocytopenia in patients scheduled for elective invasive procedures (21, 22).
Despite the Wealth of Data available on the use of thrombopoietin-receptor agonists in patients with chronic liver disease, there remains a need for a comprehensive analysis of all available evidence. Rose et al. conducted a meta-analysis to evaluate the safety and efficacy of thrombopoietin-receptor agonists in patients with severe thrombocytopenia and liver cirrhosis undergoing elective invasive procedures (23). Their findings indicated that thrombopoietin-receptor agonist administration led to an increase in platelet count and a reduction in the need for perioperative transfusions. However, their study was limited to data up to 2019, and since then, further research has yielded significant findings that warrant consideration. Additionally, their analysis did not encompass all the thrombopoietin-receptor agonist medications used in clinical practice.
2. Objectives
This review aimed to provide additional evaluation regarding the efficacy and safety of thrombopoietin-receptor agonists in chronic liver disease patients.
3. Methods
3.1. Data Search
For the purpose of performing and reporting meta-analysis data, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement requirements were adhered to. By applying the patient, intervention, comparison, and outcome (PICO) concept, the qualifying requirements were determined. The PICO structure utilized in this investigation is shown in Appendix 1 in Supplementary File. The Boolean operator was used to extract the eligibility criterion (PICO) into keywords. In order to locate relevant journals for this study, we searched the PubMed database, Google Scholar, COCHRANE, EMBASE, and Science Direct using the following keywords: Thrombopoietin receptor agonists OR TPO-RA OR eltrombopag OR romiplastim OR avatrombopag OR lusutrombopag AND (chronic liver disease) AND (platelet count OR thrombocyte count). Clinicaltrial.gov has been added as an additional source. We assess the relevant publications' references as well. The last search was run on August 20th, 2023.
3.2. Study Selection
Three authors (DAS, IDNW, and GS) carried out the study selection procedure in order to reduce the possibility of excluding potentially pertinent research. The decisions made by the first, second, and third writers were taken into consideration where there was a dispute. First, duplicate records were disposed of in order to choose studies. A screening of titles and abstracts was done to weed out papers that weren't relevant. Studies that passed the initial assessment were then reevaluated to determine whether or not they met the review's inclusion and exclusion criteria. Before any study was finally included, it underwent a rigorous quality assessment using the Cochrane Collaboration's risk-of-bias method's critical appraisal tool (24). The articles published before April 20, 2023, were all included in the current systematic review and meta-analysis. The individuals with chronic liver disease in the research were adults. Thrombopoietin-receptor agonists were the intervention under evaluation. The intervention was contrasted with a comparator (platelet transfusion or placebo). The present investigation did not include any articles classified as case reports, reviews, cadaveric or anatomic investigations, or qualitative and economic research. All papers that lacked the necessary data for doing a meta-analysis as well as any studies that included a comparison were eliminated.
3.3. Data Extraction
Studies involving individuals with chronic liver disease who received thrombopoietin-receptor agonists were considered in this analysis. The demographic of the study (chronic liver disease only and chronic liver disease mixed with non-chronic liver disease) and the usage of thrombopoietin-receptor agonist medications (avatrombopag, lusutrombopag, and eltrombopag) were the factors we used to classify the research. Thrombopoietin-receptor agonists are the intervention under review. Studies that used thrombopoietin-receptor agonists at any dose or for any length of time were included. Nonetheless, we took note of the thrombopoietin-receptor agonist dosages and durations provided for every trial. The effectiveness of thrombopoietin-receptor agonists was the main outcome that was examined in this systematic review and meta-analysis. The measurement of thrombocyte alterations (in 109/L) and the avoidance of thrombocyte transfusions are used to evaluate the effectiveness of thrombopoietin-receptor agonists. The safety of thrombopoietin-receptor agonists in patients with chronic liver disease was the secondary endpoint. The all-cause mortality, bleeding event, and thrombosis event were shown to be safe. To gather the necessary data from each publication, the writers employed an electronic data collecting form. Review Manager 5.4 was then used to handle and integrate the data. The study name, study type, population, thrombopoietin-receptor agonist dose, thrombopoietin-receptor agonist duration, sample size, age, and baseline thrombocyte count were the data items. A meta-analysis was conducted to determine the mean difference in thrombocyte levels before and after intervention, the total number of patients who did not require transfusion, and adverse events (bleeding, thrombosis, and all-cause death) in both the treatment and control groups.
3.4. Risk of Bias
The quality of every article that met the requirements for this review's eligibility was checked using a standardized critical assessment technique. Two writers separately carried out this procedure, which sought to reduce the possibility of bias in study selection (DAS and IDNW). The risk-of-bias technique developed by the Cochrane Collaboration was the critical evaluation instrument used in this review (24).
3.5. Statistical Analysis
The mean variation in thrombocyte levels between chronic liver disease patients with and without thrombopoietin-receptor agonists was combined and examined. According to Luo et al. and Wan et al. (25, 26), a calculator was used to determine the mean when the data was supplied as the median with Q1 and Q3 or range. The 95% confidence interval (CI) for the mean difference represents the effect magnitude. The analysis employed a fixed or random effect model. Values > 60% indicate significant heterogeneity. The I2 statistic was used to measure heterogeneity. It shows what percentage of the difference in observed effects across studies is attributable to the variation in real effects. Additional analyses using sensitivity and meta-regression analysis were carried out if the heterogeneity was more than 60%. Sensitivity analysis performed using the leave-one-out technique, which involves redoing the analysis after deleting one research at a time. P-values less than 0.05 were regarded as significant. Software Review Manager 5.4 was used for the meta-analysis, Stata 17.0 for Windows was used for the sensitivity analysis, and Comprehensive Meta-Analysis Software Version 3 was used for the meta-regression.
4. Results
4.1. Study Selection
We discovered a total of 187 studies through database searches and 506 studies using extra records from ClinicalTrials.gov by employing the leading search approach. Following the removal of duplicates, 665 articles were collected. After going over the titles and abstracts of 665 papers, we were left with 45 studies that were deemed relevant. Studies that did not include a comparison in the research, did not offer all the information required for this meta-analysis, or reported from the same study were eliminated. After screening and qualitative assessment, 14 papers were found and selected for the current systematic review and meta-analysis. The flow diagram for the PRISMA research is shown in Appendix 2 in Supplementary File.
4.2. Study Characteristics
We included 14 studies in this review with a total of 1529 patients for the TPO-RA group and 911 patients for the comparator group. The ADAPT 1 study was divided into ADAPT 1a (for the first arm in the ADAPT 1 study) and ADAPT 1b (for the second arm in the ADAPT 1 study). The same approach was applied to the ADAPT 2 study. The detailed data of the ADAPT study were derived from reports in ClinicalTrials.gov (7, 8). Cohort A and Cohort B studies were derived from the first arm and second arm of the article by Terrault et al. (12). The detailed data for the Cohort study were derived from ClinicalTrials.gov (13). The ELEVATE study was obtained from an article by Afdhal et al. (14), with detailed data from ClinicalTrials.gov (15). As for ENABLE studies, they were derived from an article by Afdhal et al. (16), with detailed data obtained from ClinicalTrials.gov (17, 18). The summary of findings and study characteristics can be seen in Table 1.
| Study | Type of Study | Population | Doses of TPO - RA and Comparator | Duration | Sample Size, Experiment vs Control | Age, Experiment vs Control | Baseline PLT, Experiment vs Control |
|---|---|---|---|---|---|---|---|
| ADAPT 1a (6, 7) | Global, multicenter, randomized, double - blind, placebo - controlled, parallel group | Chronic liver disease patients | 60 mg avatrombopag vs 60 mg placebo | Days 1 through 5, and 5 to 8 days prior to the scheduled procedure | 88 vs 48 samples | 55.6 ± 9.12 vs 55.1 ± 11.02, y | Less than 40 × 109/L |
| ADAPT 1b (6, 7) | Global, multicenter, randomized, double - blind, placebo - controlled, parallel group | Chronic liver disease patients | 40 mg avatrombopag vs 40 mg placebo | Days 1 through 5, and 5 to 8 days prior to the scheduled procedure | 58 vs 32 samples | 57.5 ± 10.06 vs 11.05 ± 57.8, y | Greater than or equal to 40 to less than 50 × 109/L |
| ADAPT 2a (6, 8) | Global, multicenter, randomized, double - blind, placebo - controlled, parallel group | Chronic liver disease patients | 60 mg avatrombopag vs 60 mg placebo | Days 1 through 5 | 69 vs 43 samples | 58.6 ± 14.18 vs 57.3 ± 11.98, y | Less than 40 × 109/L |
| ADAPT 2b (6, 8) | Global, multicenter, randomized, double - blind, placebo - controlled, parallel group | Chronic liver disease patients | 40 mg avatrombopag vs 40 mg placebo | Days 1 through 5 | 58 vs 33 samples | 57.9 ± 11.11 vs 59.2 ± 10.31, y | Greater than or equal to 40 to less than 50 × 109/L |
| L - PLUS 1 (9) | Multicenter, randomized, double - blind, parallelgroup, placebo - controlled, phase 3 study | Chronic liver disease patients | Lusutrombopag 3 mg vs placebo | 7 days | 48 vs 48 samples | ||
| 38 vs 41 samples for this meta - analysis | 68.9 ± 6.6 vs 66.8 ± 10.2 years | 40,900 ± 6,300 vs 39,900 ± 6,900 µL | |||||
| L - PLUS 2 (10) | multinational, phase 3, randomized, double - blind, placebo - controlled study | Chronic liver disease patients | Lusutrombopag 3 mg vs placebo | 7 days | 74 vs 73 samples | ||
| 69 vs 72 samples for this meta - analysis (day seven of drug administration) | 55.2 ± 11.6 vs 56.1 ± 11.0 years | 37.7 ± 9.0 vs 37.4 ± 7.8 × 109/L | |||||
| Takeuchi et al. 2021 (11) | Observational study | Chronic liver disease patients | Lusutrombopag vs platelet transfusion | 7 days | 10 vs 10 samples | 67.5 (36 - 86) years for total samples (n = 80) | 6.1 (1.4 - 9.3) × 104/μL for total samples (n = 80) |
| Cohort A (12, 13) | A phase II, multicentre, randomized, placebo - controlled, double - blind, parallel - group study | Chronic liver disease patients | Avatrombopag first - generation 100 mg loading dose followed by either 20 mg/day, 40 mg/day or 80 mg/day vs placebo | 7 days | 51 vs 16 samples | 54.48 ± 6.95 vs 54.2 ± 6.87, y | 40.0 (18 - 55) vs 38.0 (18 - 52) × 109/L |
| Cohort B (12, 13) | A phase II, multicentre, randomized, placebo - controlled, double - blind, parallel - group study | Chronic liver disease patients | Avatrombopag second - generation 80 mg loading dose followed by either 10 mg/day or 20 mg/day vs placebo | 7 days for 10 mg/day group, 4 days for 20 mg/day group | 42 vs 21 samples | 55.35 ± 6.1 vs 55.6 ± 6.52, y | 42.0 (18 - 57) vs 38 (20 - 55) × 109/L |
| ELEVATE (14, 15) | double - blind, randomized, placebo - controlled, phase 3 trial | Chronic liver disease patients | Eltrombopag 75 mg once daily vs placebo | 14 days | 131 vs 132 samples | 51.6 ± 11.04 vs 53.5 ± 11.78, y | 40 (3 - 55) vs 40 (8 - 70) Gi/L |
| ENABLE 1 (16, 17) | Randomised, placebo - controlled, multi - centre study | Confirmed HCV infection | Eltrombopag vs placebo | 24 or 48 weeks according to the HCV genotype | 419 vs 212 patients | 51.9 ± 8.41 vs 52.1 ± 8.35, y | 133.00 (64.00 - 509.00) vs 128.00 (84.00 - 521.00) Gi/L |
| ENABLE 2 (16, 18) | Randomised, placebo - controlled, multi - centre study | Confirmed HCV infection | Eltrombopag vs placebo | 24 or 48 weeks according to the HCV genotype | 468 vs 240 patients | 52.4 ± 8.61 vs 52.0 ± 9.15, y | 56.85 ± 13.311 vs 56.56 ± 13.571 Gi/L |
| NCT02227693 (19) | Phase 2, randomized, double - blind, placebo - controlled, parallel group study | Chronic liver disease patients | Avatrombopag 20 mg, 40 mg or 60 mg) vs placebo | 5 days | 28 vs 11 patients | 63.71 ± 8.32 vs 71.1 ± 8.49, y | 36.64 ± 8.42 vs 40.59 ± 7.024 × 109/L |
| Tateishi et al. 2019 (20) | Multicenter, randomized, double - blind, parallelgroup, placebo - controlled, phase 2b | Chronic liver disease patients | Lusutrombopag (2 mg, 3 mg, or 4 mg) vs placebo | 7 days | 46 vs 15 patients | 65.98 ± 8.58 vs 70.9 ± 8.6, y | 40.69 ± 9.51 vs 41.8 ± 6.1 × 103/µL |
Abbreviations: PLT, platelet; TPO-RA, thrombopoietin receptor agonists.
a For two to nine weeks, Eltrombopag was given to each patient at a dose that increased based on their platelet response (25, 50, 75, or 100 mg once daily). Following that, the patients were split into two groups: One for eltrombopag (which was administered at the same dose as during the start phase) and the other for placebo.
b All data presented as mean ± SD, unless indicated otherwise.
c Data presented as median (range).
4.3. Efficacy of TPO-receptor Agonist in Chronic Liver Disease Patients
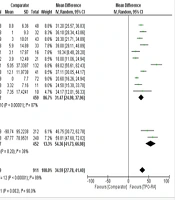
The efficacy of TPO-RA is determined by changes in thrombocyte levels and the likelihood of avoiding platelet transfusion. The use of TPO-RA significantly increases thrombocyte levels. Based on a random effect model (I2 = 89%; χ2 = 105.66; P < 0.00001), the pooled mean difference in thrombocyte levels between the TPO-RA group and the comparator (placebo and platelet transfusion) was 34.59 × 109/L (P < 0.00001; 95% CI: 27.78 to 41.40) (Figure 1).
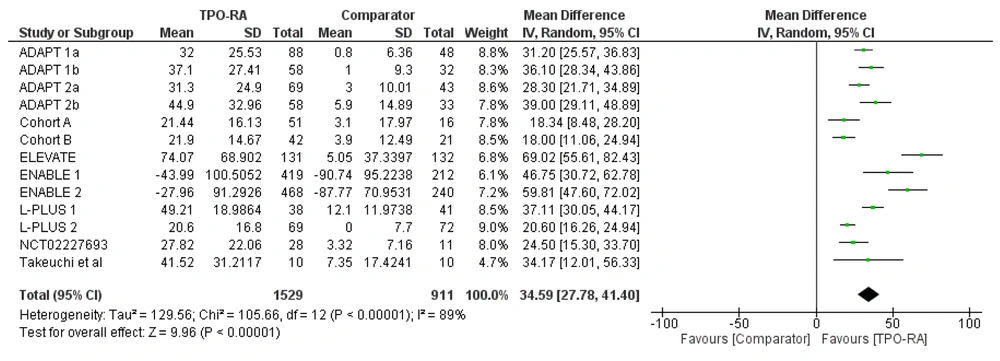
The subgroup analysis for the use of TPO-RA in increasing thrombocyte levels was also performed based on the population of the study. Two studies (ENABLE 1 and ENABLE 2) involved patients with confirmed Heoatitis C virus (HCV) infection, irrespective of the CLD status of patients. Therefore, subgroup analysis between CLD-only population studies and ENABLE studies was performed. Based on the analysis, the use of TPO-RA increased thrombocyte levels significantly in both the CLD-only subgroup and ENABLE studies (CLD and without CLD). The use of TPO-RA increased platelet levels with a pooled mean difference of 31.47 × 109/L (P < 0.00001; 95% CI: 24.98 to 37.96) in the CLD-only subgroup and 54.36 × 109/L (P < 0.00001; 95% CI: 41.73 to 66.98) for ENABLE studies (Figure 2).
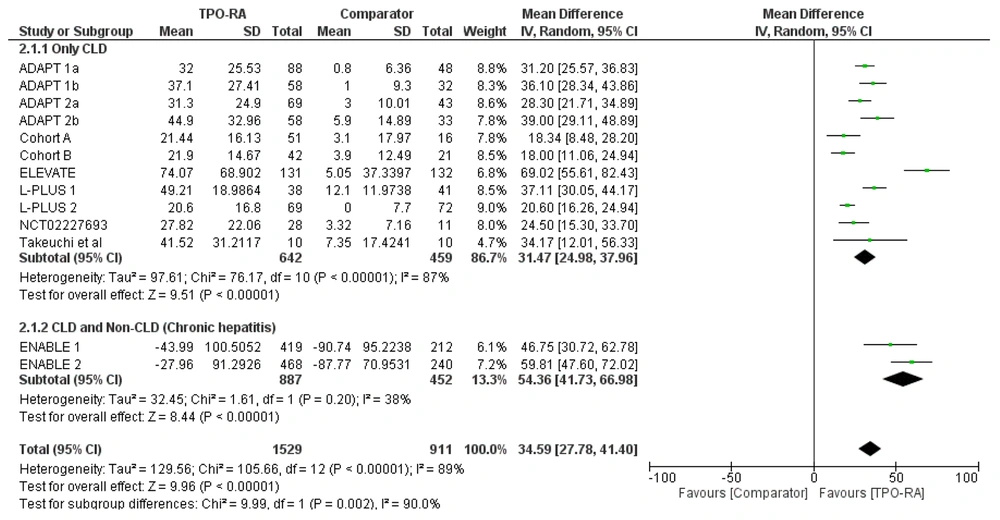
Subgroup analysis was also performed based on the TPO-RA drugs used (avatrombopag, lusutrombopag, and eltrombopag). All drugs significantly increased thrombocyte levels, with the highest pooled mean difference observed in the eltrombopag group (59.21 x 109/L [P < 0.00001; 95% CI: 47.45 to 70.97]). The pooled mean difference for avatrombopag was 27.93 x 109/L (P < 0.00001; 95% CI: 22.3 to 33.56) and 29.7 x 109/L (P < 0.00001; 95% CI: 16.1 to 43.31) for lusutrombopag (Figure 3).
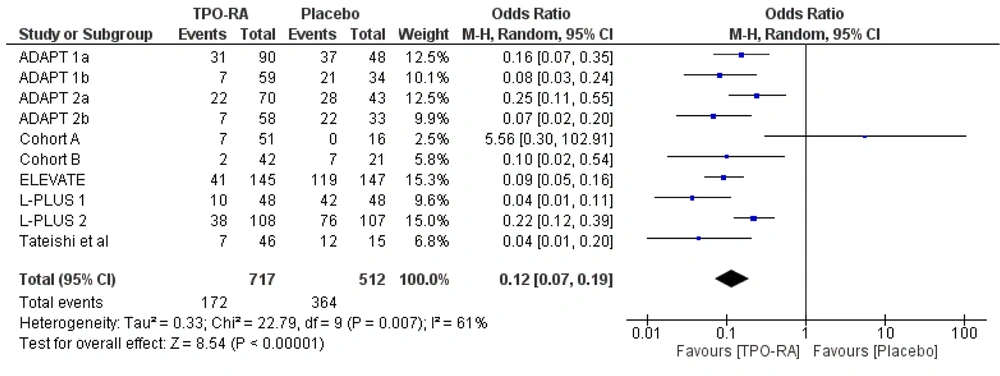
Another outcome assessed for the efficacy of TPO-RA was the likelihood of avoiding pre-operative platelet transfusion and up to seven days following the scheduled procedure. Ten studies analyzed the use of TPO-RA to avoid pre-operative platelet transfusion and up to seven days following the scheduled procedure. The use of TPO-RA pre-procedure reduced the likelihood of pre-operative platelet transfusion and up to seven days following the scheduled procedure by 88%, with a pooled OR of 0.12 (95% CI: 0.07 to 0.19), compared to placebo (Figure 4).
4.4. Safety of TPO-receptor Agonist in Chronic Liver Disease Patients
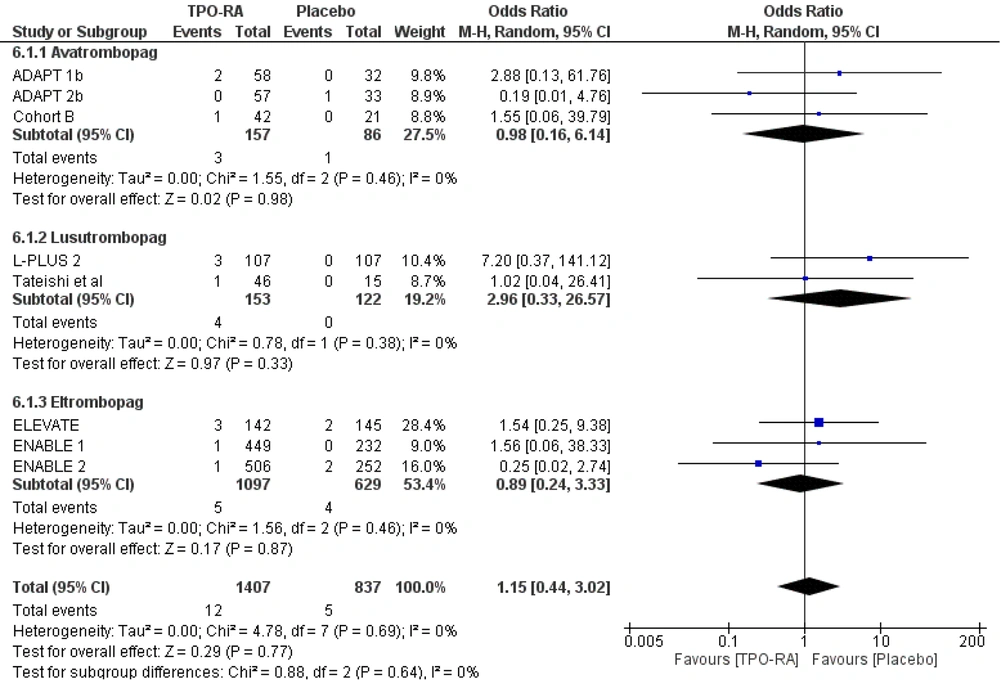
The safety of TPO-RA was assessed based on the odds for mortality, bleeding events, and thrombosis events. The use of TPO-RA was not associated with all-cause mortality (OR 1.15, 95% CI 0.44 to 3.02, P = 0.77). The same results were obtained even after subgroup analysis based on the drugs used, indicating that avatrombopag, lusutrombopag, or eltrombopag was not associated with an increased likelihood of all-cause mortality (Figure 5).
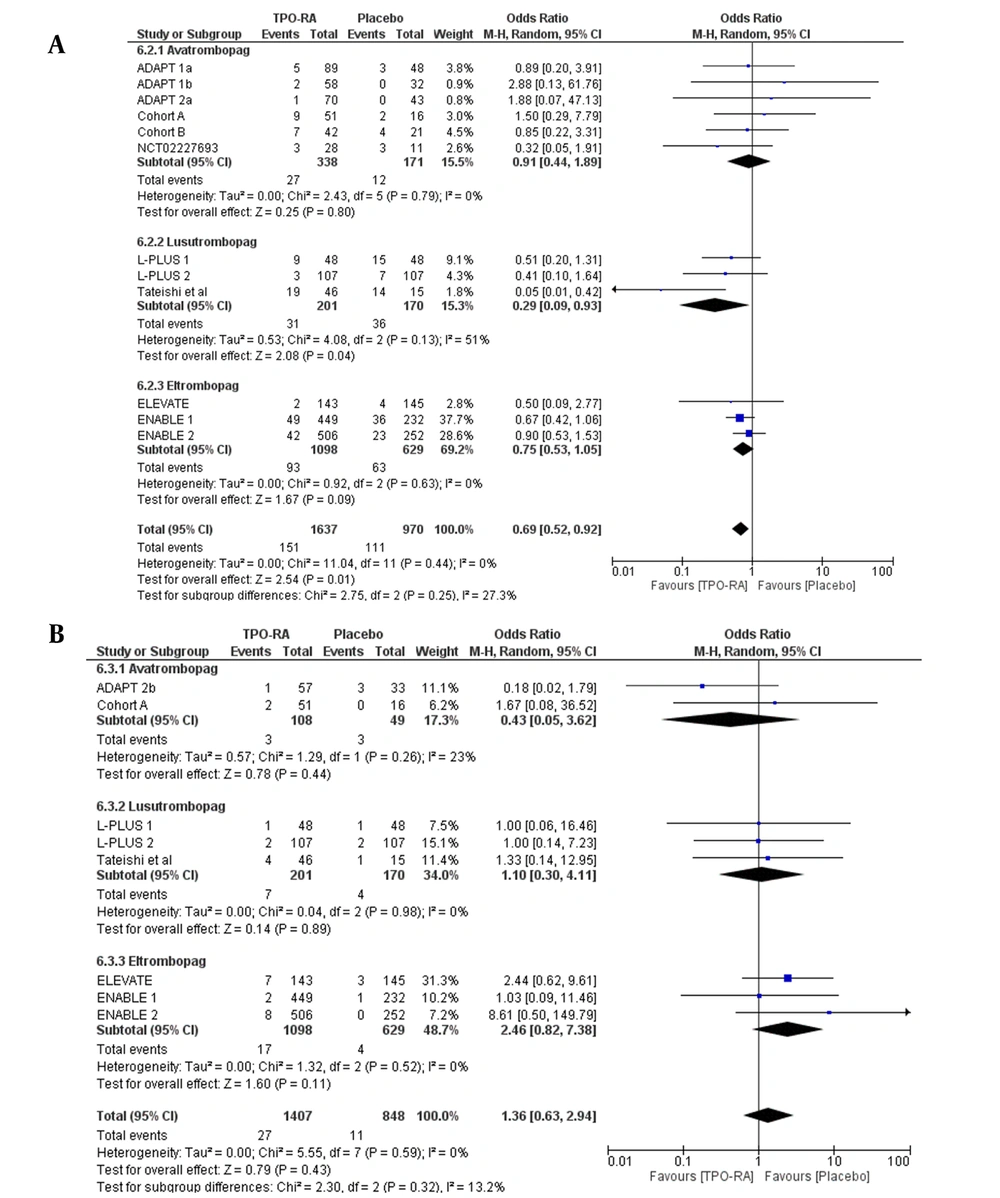
Reduced odds for bleeding events were observed with the use of TPO-RA, compared to placebo, with an OR of 0.69 (95% CI 0.52 to 0.92, P = 0.01). This effect was observed in patients who received lusutrombopag, but not for avatrombopag and eltrombopag (P > 0.05) (Figure 6A). As for thrombosis events, the use of TPO-RA was not associated with an increase in thrombosis events, with a pooled OR of 1.36 (95% CI: 0.63 to 2.94). This result was the same even after subgroup analysis by the drugs used (Figure 6B).
4.5. Meta-Regression and Sensitivity Analysis
There was a significant amount of heterogeneity (I2 = 89%) regarding the change in thrombocyte levels after TPO-RA treatment. The population may be able to explain the heterogeneity, according to a meta-regression study of duration (less than 14 days or more than 14 days) or population (both CLD-only and non-CLD-only) with mean difference in thrombocyte levels (Appendix 3 in Supplementary File). Except for the ENABLE 1 and ENABLE 2 investigations, all of the studies included a population with CLD (17, 18). Subsequent leave-one-out sensitivity analysis of the thrombocyte level change after TPO-RA revealed that no study was accountable for the heterogeneity of thrombocyte level changes (Appendix 4 in Supplementary File).
5. Discussion
This meta-analysis and systematic review unveiled several noteworthy findings. When compared to placebo or thrombocyte transfusion, the use of TPO-RA substantially elevated thrombocyte levels in individuals with CLD. Notably, all TPO-RA medications, including avatrombopag, lusutrombopag, and eltrombopag, demonstrated efficacy in this regard. Furthermore, TPO-RA administration reduced the likelihood of requiring thrombocyte transfusion prior to elective operations. Moreover, TPO-RA was associated with a decreased risk of bleeding events in the CLD population, although it did not influence mortality or thrombosis events in this cohort.
While hepatocytes serve as the main producers of thrombopoietin (TPO), a small quantity of this hormone is also synthesized by the kidney and bone marrow (1). Megakaryocytes and thrombocytes possess receptors to which circulating TPO binds. This interaction prompts megakaryocytes and thrombocytes to proliferate, enlarge, and undergo differentiation by inhibiting apoptosis. Thrombocytopenia arises due to diminished TPO concentration in advanced liver disease (27, 28).
Eltrombopag and romiplostim were among the first TPO-RAs to be developed. Subsequently, avatrombopag and lusutrombopag, two nonpeptide agonists, have obtained approval for use in patients with advanced liver disease who experience thrombocytopenia and are scheduled for surgical procedures. The ADAPT trials (ADAPT 1 and ADAPT 2) demonstrated an increase in thrombocyte levels when avatrombopag was administered at doses of 40 mg or 60 mg five days prior to elective surgeries (6). Additionally, based on findings from the L-PLUS-1 and L-PLUS-2 trials, lusutrombopag has also received approval in the US (9, 10).
The current review revealed a significant increase in thrombocyte levels in patients with CLD following the administration of TPO-RAs. However, it's important to note that two studies (ENABLE 1 and ENABLE 2) focused on individuals with confirmed HCV infection, regardless of their CLD status, thereby including patients without CLD. Consequently, these studies enrolled patients without CLD as well (17, 18). In addition to assessing the impact of TPO-RA on thrombocyte levels in both CLD and non-CLD populations, this review also evaluated the efficacy of TPO-RAs specifically in CLD-only studies by excluding the ENABLE 1 and ENABLE 2 trials through subgroup analysis. Interestingly, similar results were obtained, demonstrating a significant increase in thrombocyte levels in the CLD-only population with the use of TPO-RAs. Furthermore, all TPO-RA medications, including avatrombopag, lusutrombopag, and eltrombopag, were found to elevate thrombocyte levels, with eltrombopag showing the highest efficacy.
Another aspect of TPO-RA efficacy in the CLD population is evaluated through its ability to obviate the need for thrombocyte transfusion prior to invasive procedures. While not all CLD patients with thrombocytopenia undergo treatment for their low platelet count, correcting thrombocytopenia in certain CLD patients scheduled for invasive procedures is crucial before proceeding with elective surgery. Platelet transfusions are commonly employed to manage platelet counts during the perioperative period in thrombocytopenic patients, as low platelet counts have been linked to an increased risk of bleeding (4, 29). The recommended target for thrombocyte levels varies depending on the risk associated with the procedure being performed, categorized as low risk (e.g., paracentesis, esophagogastroduodenoscopy, colonoscopy, etc.), moderate risk (e.g., percutaneous liver biopsy, thoracentesis, percutaneous endoscopic gastrostomy or jejunostomy tube placement, etc.), and high-risk procedures (intracranial and spinal procedures). The recommended thrombocyte thresholds are ≥ 20,000/μL, ≥ 50,000/μL, and ≥ 100,000/μL for low, moderate, and high-risk procedures, respectively.
The utilization of thrombocyte transfusion to augment thrombocyte levels is hindered by several drawbacks, with medical harm being the most significant concern associated with transfusion. Potential complications include transfusion reactions, hypersensitivity reactions, acute lung injury, circulatory overload, infections, and hemolysis (4). Moreover, a longer-term issue arising after multiple thrombocyte transfusions is alloimmunization, characterized by the production of antiplatelet antibodies. This mechanism contributes to the refractoriness of thrombocytopenia despite transfusion. One approach to address this issue is the use of matched platelets; however, this strategy is constrained by limited availability. A more substantial concern arises in patients slated for liver transplantation or other major procedures who develop alloimmunization. These individuals may necessitate extensive transfusions, and alloimmunization could potentially preclude them from undergoing such procedures. Additionally, the cost and logistical challenges associated with transfusion represent further issues (3). Multiple studies have consistently demonstrated that TPO-Ras obviate the necessity for thrombocyte transfusions prior to treatment. The ADAPT trials yielded favorable outcomes in terms of averting the need for thrombocyte transfusions or rescue interventions for bleeding (6-8). Similarly, the L-PLUS-1 and L-PLUS-2 investigations indicated that lusutrombopag achieved comparable results. These studies revealed that in patients with thrombocytopenia and chronic liver disease scheduled for invasive procedures, lusutrombopag reduced the requirement for platelet transfusions (9, 10). According to the findings of the current meta-analysis, the use of TPO-RA generally decreases the risk of platelet transfusions before and up to seven days after planned treatment by 88%. Concerns regarding the safety of TPO-RAs have centered around the occurrence of portal vein thrombosis (PVT) (30). Although eltrombopag has been utilized in hepatitis C patients receiving interferon, its use in liver disease patients is not routine due to instances of PVT and hepatotoxicity (14, 16). Notably, the ADAPT studies excluded patients at high risk of PVT and reported favorable overall safety profiles. The incidence of thromboembolic events in the ADAPT studies did not differ significantly from that in the placebo group. Similar results were observed in the L-PLUS-1 and L-PLUS-2 studies, where the thrombosis rate did not vary from the placebo groups (9, 10). The current review findings indicate that the all-cause mortality rate and thrombotic events in patients receiving TPO-RAs did not differ from those in the placebo group. Moreover, bleeding events were reduced in the TPO-RA groups. While thromboembolic events are a significant safety concern associated with TPO-RA drugs, it's essential to consider other adverse effects as well. Avatrombopag, for instance, can lead to various adverse effects, including bleeding problems, infections, and headaches. Headache is the most common adverse effect of avatrombopag, accounting for 31% of incidences. Bleeding adverse effects such as epistaxis, gingival bleeding, and petechiae may also occur. Upper respiratory tract infections and nasopharyngitis should also be considered when using avatrombopag. In the case of eltrombopag, adverse events to monitor include increased liver enzymes (elevations in alanine aminotransferase and aspartate aminotransferase), elevated bilirubin levels, nausea, and diarrhea. Romiplostim, on the other hand, may cause musculoskeletal problems such as arthralgia, myalgia, shoulder pain, and pain in extremities, as well as paresthesia, abdominal pain, and dyspepsia (31, 32). To the best of the authors' knowledge, only one meta-analysis has been conducted to assess the efficacy and safety of TPO-RA use in individuals with chronic liver disease (23). The main objective of this study was to determine the number of patients who avoided platelet transfusion during the peri-procedural phase. Secondary outcomes included the rate of major adverse events such as bleeding, thrombotic events, and mortality risk, as well as the weighted mean difference (WMD) in platelet count between pre-procedure and baseline. The meta-analysis concluded that TPO-RA improved thrombocyte counts, reduced the need for transfusions, and exhibited no significant side effects compared to placebo. The latest meta-analysis included more references and encompassed a larger patient population than its predecessor. As the primary outcomes differed, the studies included in this current meta-analysis are more extensive. Additionally, this meta-analysis focused on analyzing the efficacy and safety of each TPO-RA drug through subgroup analysis, whereas the previous one only subgrouped studies for the primary outcome (23). Consequently, the current meta-analysis provides more comprehensive data for each drug used in the primary outcome and safety assessments. The current review is subject to several limitations that warrant consideration. Further analysis is required to evaluate the duration of thrombocytes sustained within the targeted range. Moreover, the safety issue of thrombosis in higher-risk populations necessitates additional evaluation, particularly since previous studies excluded this demographic. Additionally, other adverse events associated with TPO-RA drugs, beyond thromboembolic events and bleeding events, should be thoroughly examined. It would also be beneficial to explore thresholds for platelet transfusion in various clinical contexts, including preoperative assessment, invasive procedures, or bleeding events. Therefore, further safety evaluation is imperative before considering TPO-RA for use in a broader population.
5.1. Conclusions
In conclusion, TPO-RA use effectively increases thrombocyte levels in patients with chronic liver disease and reduces the likelihood of requiring thrombocyte transfusion preoperatively. Moreover, TPO-RAs demonstrate safety in terms of mortality and thrombosis risk.