1. Background
The liver is the primary organ responsible for regulating essential biochemical processes, including metabolism, immunity, and endocrine functions, which contributes to its complex involvement in liver diseases (1). Liver fibrosis, a critical stage in the progression of various chronic liver diseases to cirrhosis (2), is commonly attributed to two types of chronic liver damage: Hepatotoxic and bile duct damage, such as those caused by chronic viral infections (3, 4), alcohol consumption (5), metabolic syndrome (6), and cholestasis (7). Current epidemiological data suggest that liver diseases are linked to two million deaths annually, accounting for 4% of all deaths worldwide (8). However, this statistical data might underestimate the actual prevalence of liver diseases, given the disease's insidious nature (9, 10).
Liver fibrosis is characterized by a physiological reaction aimed at repairing liver damage, which results in a network of abnormally distributed extracellular matrix across the damaged sites. The molecular mechanisms underlying liver fibrosis encompass chronic hepatocyte injury, impairment of the epithelial or endothelial barrier, production of inflammatory cytokines, recruitment of bone marrow-derived inflammatory cells, release of TGF-β by macrophages, and activation of hepatic myofibroblasts to secrete type I collagen (COL1A1), leading to fibrous scarring. Key immune cells involved in the development of liver fibrosis include sinusoidal endothelial cells (11), macrophages, and myofibroblasts (12). Liver macrophages, comprising Kupffer cells (resident macrophages) and monocyte-derived macrophages, constitute 80% of the body's total macrophage population (13). These macrophages are essential in maintaining liver homeostasis and play a crucial role in liver disease progression. They remove pathogens or cellular debris and maintain immune tolerance under normal conditions, playing a pivotal role in initiating and perpetuating inflammation in response to injury. In addition, they contribute to addressing inflammation and fibrosis by degrading the extracellular matrix and releasing anti-inflammatory cytokines (14). Experimental models of liver fibrosis have demonstrated that hepatic macrophages can exhibit a dual function, either promoting or mitigating the excessive deposition of the extracellular matrix (15).
CiteSpace is a document visualization analysis application developed by Professor Chen Chaomei at Drexel University in the United States (16). VOSviewer is a Java-based software available for free. It was created in 2009 by Van Eck and Waltman from the Centre for Science and Technology Studies (CWTS) at Leiden University in the Netherlands (17). While academic researchers have published bibliometric studies on macrophages in contexts such as osteoarthritis (18) and cardiovascular disease (19), there has not yet been a similar analysis focusing on macrophages in liver fibrosis. These bibliometric analysis tools enable the quantitative analysis of scientific literature to identify specific research trends, thereby assisting researchers and institutions in gaining a clearer understanding of the current state of research and potential future directions (20).
2. Objectives
In this bibliometric analysis, we have investigated the trends in research concerning the regulation of liver fibrosis by macrophages through a systematic literature review.
3. Methods
3.1. Data Collection
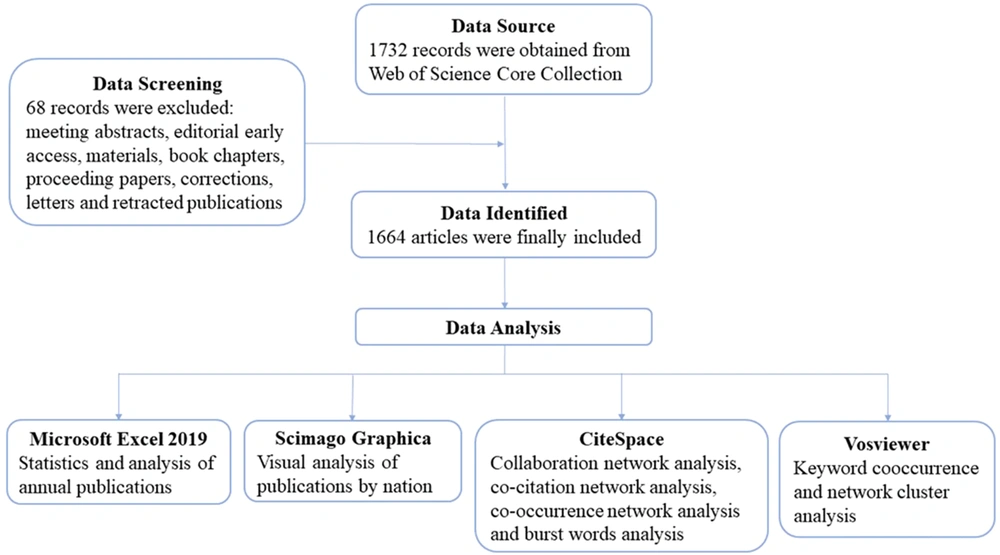
This study gathered scientific publications on macrophages in liver fibrosis from the Web of Science Core Collection (WoSCC) database, covering the period from 1 January 2007 to 8 October 2023. Two independent researchers conducted the literature search, and the data extraction process was carried out on 8 October 2023. The search terms used were TS=("hepatic fibrosis" or "liver fibrosis") and TS=(macrophage or "Kupffer cell"). Only publications relevant to these topics, and with complete information including title, abstract, keywords, authors, and institutions, were exported with the option “Full Record and References.” The files were saved in download_txt format for further analysis. Additionally, this study excluded publications such as meeting abstracts, editorial materials, early access articles, proceeding papers, book chapters, corrections, letters, and retracted publications. A total of 1 664 eligible articles were identified and imported into bibliometric analysis tools for further analysis (Figure 1).
3.2. Data Analysis
Microsoft Office Excel 2019 was utilized for data management and to analyze and visualize the annual distribution of articles. Bibliometric analysis software was employed to summarize the existing studies and to explore the knowledge structure within the field. CiteSpace (version 6.1.R6) and Scimago Graphica (version 1.0.34.0) were used to visualize the collaboration network among countries. CiteSpace also facilitated the analysis of institutions and the identification of top references and keywords with the most significant citation bursts. VOSviewer (version 1.6.19) was used for co-authorship and co-occurrence analyses.
4. Results
4.1. Annual Analysis of Publications
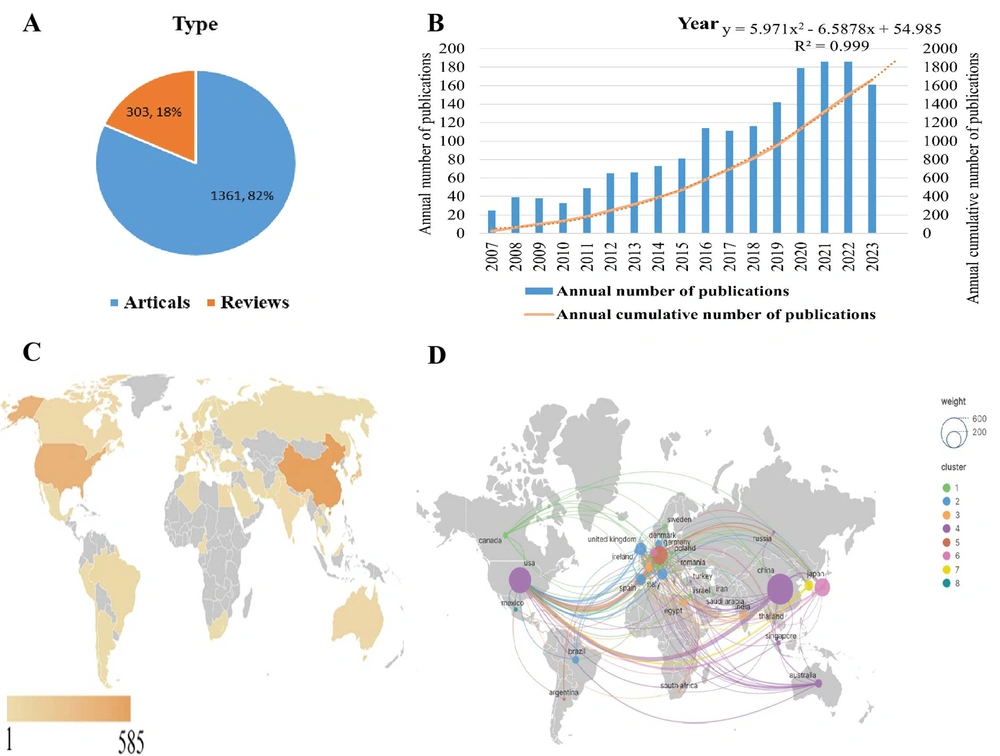
According to the search criteria, a total of 1 664 publications related to macrophage-mediated liver fibrosis research from 2007 to 2023 were identified in the WoSCC, comprising 1 361 (81.8%) original articles and 303 (18.2%) reviews (Figure 2A). The annual and cumulative publications on macrophage-mediated hepatic fibrosis have shown a steady and gradual increase (Figure 2B). The cumulative number of publications reached 1 503 in 2022. Based on the prediction curve, the literature on macrophages in liver fibrosis is expected to continue growing, with an anticipated cumulative total of 1 983 by the end of 2023 (R2 = 0.999).
4.2. Distribution Characteristics of Countries/Regions
The analysis revealed that China, the USA, Germany, Japan, and Italy were the most productive countries, as shown in Figure 2C. Meanwhile, Germany, England, the USA, France, and Japan held the highest centrality, indicating they were the most influential countries in the research field of macrophages and liver fibrosis (Table 1). China led with 585 publications, followed by the USA with 414 articles, and Germany with 210. In terms of centrality, Germany was at the forefront with a centrality of 0.41, followed by England (0.34) and the USA (0.33).
| Rank | Country (Publications) | Country (Centrality) | Institution (Publications) | Institution (Centrality) |
|---|---|---|---|---|
| 1 | China (585) | Germany (0.41) | RWTH Aachen University (80) | University of California San Diego (0.14) |
| 2 | USA (414) | England (0.34) | RWTH Aachen University Hospital (69) | Chinese Academy of Sciences (0.13) |
| 3 | Germany (210) | USA (0.33) | University of California System (67) | RWTH Aachen University Hospital (0.12) |
| 4 | Japan (200) | France (0.14) | Harvard University (44) | University of Bonn (0.12) |
| 5 | Italy (80) | Japan (0.12) | University of California San Diego (44) | University of California System (0.11) |
| 6 | England (79) | Spain (0.11) | Chinese Academy of Sciences (39) | Helmholtz Association (0.11) |
| 7 | Spain (72) | Switzerland (0.11) | Harvard Medical School (39) | Harvard Medical School (0.1) |
| 8 | South Korea (64) | Australia (0.09) | Anhui Medical University (36) | National Institutes of Health (0.1) |
| 9 | France (47) | Austria (0.09) | Centro de Investigación Biomédica en Red (36) | Icahn School of Medicine at Mount Sinai (0.1) |
| 10 | Australia (45) | China (0.07) | Institut national de la santé et de la recherche médicale (36) | University of London( 0.07) |
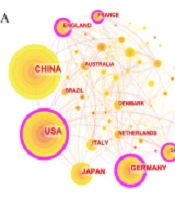
The network analysis of cooperative relations between countries using Scimago Graphica ( Figure 2D) included countries with more than one publication. The node size denotes the quantity of published articles, and the thickness of the connection lines between countries reflects the intensity of their collaboration. A larger number of nodes indicate more publications and a thicker link signifies stronger cooperation. This analysis divided the collaborations into 8 clusters based on the intensity of collaboration. China and the USA featured as the nodes with the largest size, and their connection was the thickest, indicating numerous publications and close cooperation between these two countries.
A, Types of publications collected; B, Annual number and annual cumulative number of publications in liver fibrosis associated with macrophage research from 2007 to 2023 publication counts analysis; C, World map of publications in different countries; D, The network of cooperative relations between countries by Scimago Graphics.
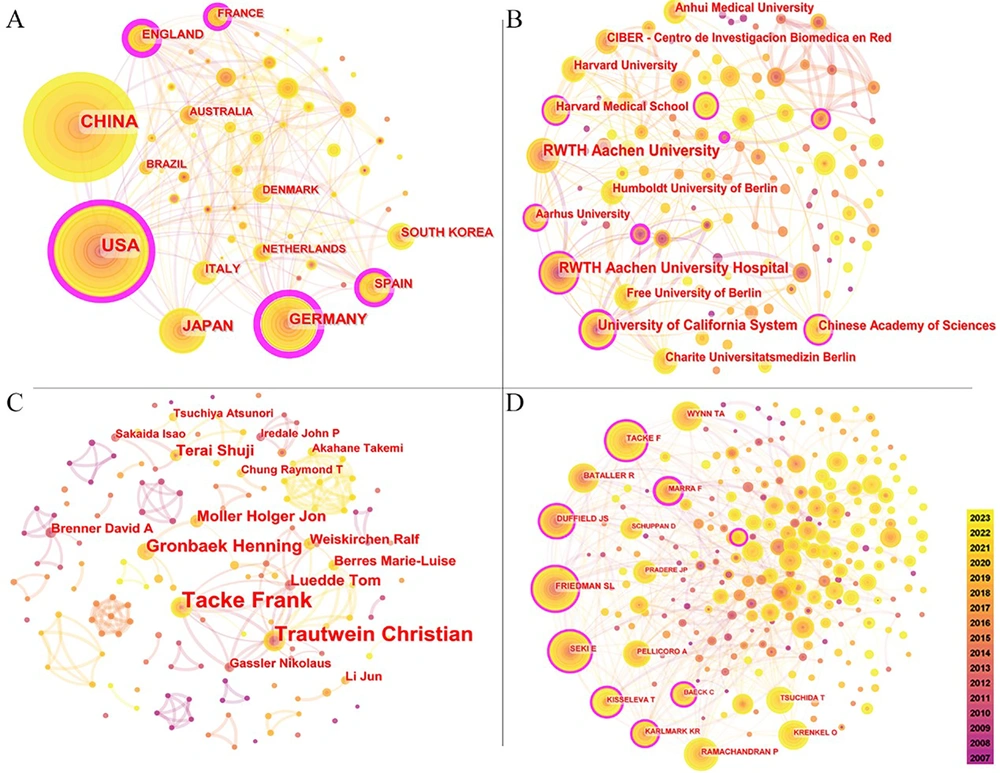
The network of cooperative relations between countries consisted of 58 nodes and 199 links (Figure 3A). The size of each node indicates the frequency of co-occurrences, while the links represent the relationships of co-occurrence. From 2007 to 2023, the color of the nodes and lines transitioned from purple to yellow. Nodes with purple outer rims signify high centrality (a node is deemed crucial when its centrality exceeds 0.1), and thicker outer rims indicate greater centrality.
4.3. Institution Analysis
The co-institution network map included 120 nodes and 228 links (Figure 3B). The top five institutions by publication count were RWTH Aachen University (n = 80), RWTH Aachen University Hospital (n = 69), University of California System (n = 67), Harvard University (n = 44), and University of California San Diego (n = 44) (Table 1). Regarding global influence, the University of California San Diego held the top position with a centrality of 0.14, followed by the Chinese Academy of Sciences (0.13), RWTH Aachen University Hospital (0.12), University of Bonn (0.12), and University of California System (0.11).
4.4. Authors Analysis
The visual network of co-authors comprised 163 nodes and 241 links (Figure 3C). Frank Tacke from Charité University Hospital (Germany) emerged as the most prolific author with 49 publications, followed by Christian Trautwein from RWTH Aachen University Hospital (Germany) with 33 publications, and Henning Grønbæk from Aarhus University Hospital (Denmark) with 19 publications (Appendix 1 in Supplementary File). Frank Tacke, Christian Trautwein, Ralf Weiskirchen, Tom Luedde, Detlef Schuppan, and Marie-Luise Berres, all from Germany, were among the top 10 most prolific authors. The network of cited authors included 252 nodes and 636 links (Figure 3D), with Scott L. Friedman ranking first with 427 citations, followed by Frank Tacke (n = 327) and Ekihiro Seki (n = 326) (Table 2).
| Rank | Author | Publications | Institution (Country) | Cited Author | Citations Counts | Institution (Country) |
|---|---|---|---|---|---|---|
| 1 | Frank Tacke | 49 | Charite University Hospital (Germany) | Friedman Scott L | 427 | Icahn School of Medicine at Mount Sinai (USA) |
| 2 | Trautwein Christian | 33 | RWTH Aachen University Hospital (Germany) | Frank Tacke | 327 | Charite University Hospital (Germany) |
| 3 | Henning Gronbaek | 19 | Aarhus University Hospital (Denmark) | Ekihiro Seki | 326 | Cedars-Sinai Medical Center (USA) |
| 4 | Ralf Weiskirchen | 14 | RWTH University Hospital Aachen (Germany) | Wynn Thomas A | 289 | Pfizer NIH National Institute of Allergy & Infectious Diseases (NIAID) (USA) |
| 5 | Moller Holger Jon | 14 | Aarhus University (Denmark) | Ramachandran Prakash | 266 | University of Edinburgh (England) |
| 6 | Terai Shuji | 13 | Niigata University (Japan) | Bataller Ramon | 264 | University Of Pittsburgh (USA) |
| 7 | Luedde Tom | 12 | Heinrich Heine University Dusseldorf (Germany) | Duffield Jeremy S | 254 | Prime Medicine (USA) |
| 8 | Schuppan Detlef | 12 | Johannes Gutenberg University of Mainz (Germany) | Tatiana Kisseleva | 197 | University California San Diego (USA) |
| 9 | Li Jun | 10 | Shanghai Institute of Technology (China) | Karlin Raja Karlmark | 185 | Aix-Marseille University (France) |
| 10 | Berres Marie-Luise | 10 | RWTH Aachen University Hospital (Germany) | Marra Fabio | 184 | University of Florence (Italy) |
4.5. Journal Analysis
The 1 664 publications related to macrophages and liver fibrosis were distributed across 528 journals. Table 3 highlights that hepatology was the leading journal in this field, publishing 77 articles and receiving 9 030 citations. Journal co-citation analysis played a crucial role in identifying the interconnectedness of various journals within a specific field. Hepatology emerged as both the most prolific and the most frequently co-cited journal, with a co-citation count of 1 423.
| Rank | Journal | Publications | Citations | Rank | Journal | Co-citation Counts |
|---|---|---|---|---|---|---|
| 1 | Hepatology | 77 | 9030 | 1 | Hepatology | 1423 |
| 2 | Frontiers in Immunology | 65 | 1917 | 2 | Journal of Hepatology | 1284 |
| 3 | Journal of Hepatology | 50 | 5439 | 3 | Gastroenterology | 1072 |
| 4 | International Journal of Molecular Sciences | 50 | 963 | 4 | The Journal of Clinical Investigation | 1058 |
| 5 | Plos One | 44 | 2123 | 5 | Plos One | 875 |
| 6 | Scientific Reports | 38 | 1092 | 6 | Proceedings of the National Academy of Sciences (PNAS) | 813 |
| 7 | World Journal of Gastroenterology | 28 | 1086 | 7 | Journal of Biological Chemistry | 773 |
| 8 | Cells | 27 | 1159 | 8 | The Journal of Immunology | 769 |
| 9 | American Journal of Physiology-Gastrointestinal and Liver Physiology | 23 | 565 | 9 | Gut | 724 |
| 10 | Liver International | 22 | 376 | 10 | Nature | 651 |
4.6. Cited References Analysis
Citation analysis serves as an important tool for assessing the influence of highly cited literature, with citation frequency indicating the impact of an article within a research area. An analysis of the ten most-cited papers on macrophages and liver fibrosis, published between 2012 and 2021, revealed a mix of three articles and seven reviews. The three top-cited references were all published in 2017, with two references receiving over 100 citations each (Appendix 2 in Supplementary File). These publications are considered seminal works in the study of liver fibrosis and macrophages. The analysis focused on the first authors and corresponding authors, revealing that Frank Tacke was a significant contributor, authoring four of the top ten highly cited papers.
4.7. Keyword Analysis
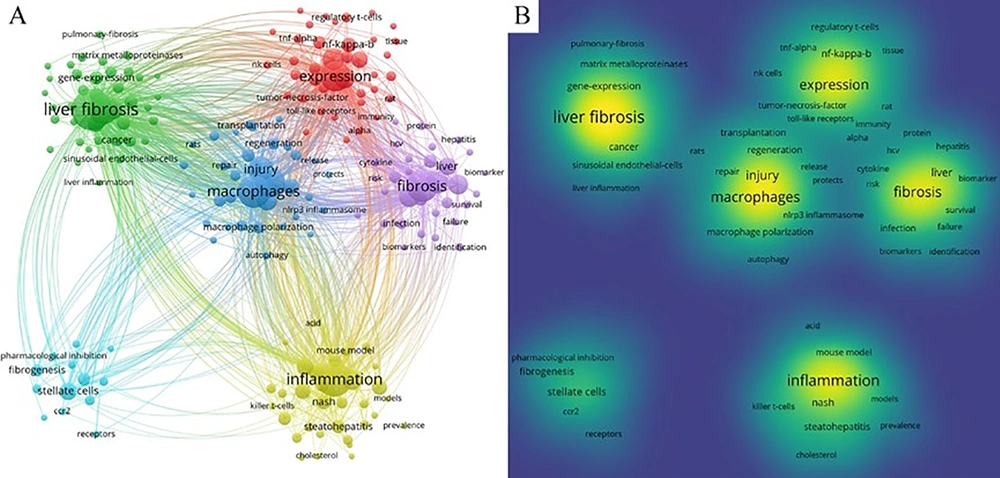
VOSviewer (version 1.6.18) facilitated keyword co-occurrence and network cluster analysis, identifying a total of 5 851 keywords. To minimize the influence of infrequent keyword occurrences, we set a threshold of “minimum number of occurrences of a keyword ≥ 20”. Consequently, 295 keywords surpassed this threshold and were organized into six clusters, representing the main research directions or areas within the study of liver fibrosis and macrophages. The keyword co-occurrence network (Figure 4A) illustrates that a thicker connection between nodes signifies a higher frequency of two keywords appearing together. The size of a node represents the frequency of keyword co-occurrences, while the varied colors denote different clusters, and the links depict the co-occurrence relationships. Specifically, Cluster 1 (red) featured keywords such as “expression” (335 occurrences), “fibrosis” (369 occurrences), “disease” (183 occurrences), and “Kupffer cell” (179 occurrences). Cluster 2 (green) included “hepatic stellate cells” (326 occurrences), “macrophage” (131 occurrences), “stellate cells” (111 occurrences), and “hepatocellular carcinoma” (100 occurrences). Cluster 3 (blue) listed “liver fibrosis” (603 occurrences), “macrophages” (398 occurrences), “activation” (327 occurrences), and “injury” (225 occurrences). Cluster 4 (yellow) contained “inflammation” (446 occurrences), “mice” (157 occurrences), “nonalcoholic steatohepatitis” (154 occurrences), and “oxidative stress” (107 occurrences). Cluster 5 (purple) held “fibrosis” (369 occurrences), “disease” (183 occurrences), “cirrhosis” (161 occurrences), and “cells” (144 occurrences). Cluster 6 (cyan) included “stellate cells” (111 occurrences), “differentiation” (641 occurrences), “fibrogenesis" (57 occurrences), and "recruitment" (39 occurrences). Additionally, the keywords with high frequency were prominently displayed in the density visualization map of keywords (Figure 4B). The size of the nodes and their density are directly related to the frequency of co-citation. The top 20 occurring keywords and their frequencies are detailed in Appendix 3 (in Supplementary File).
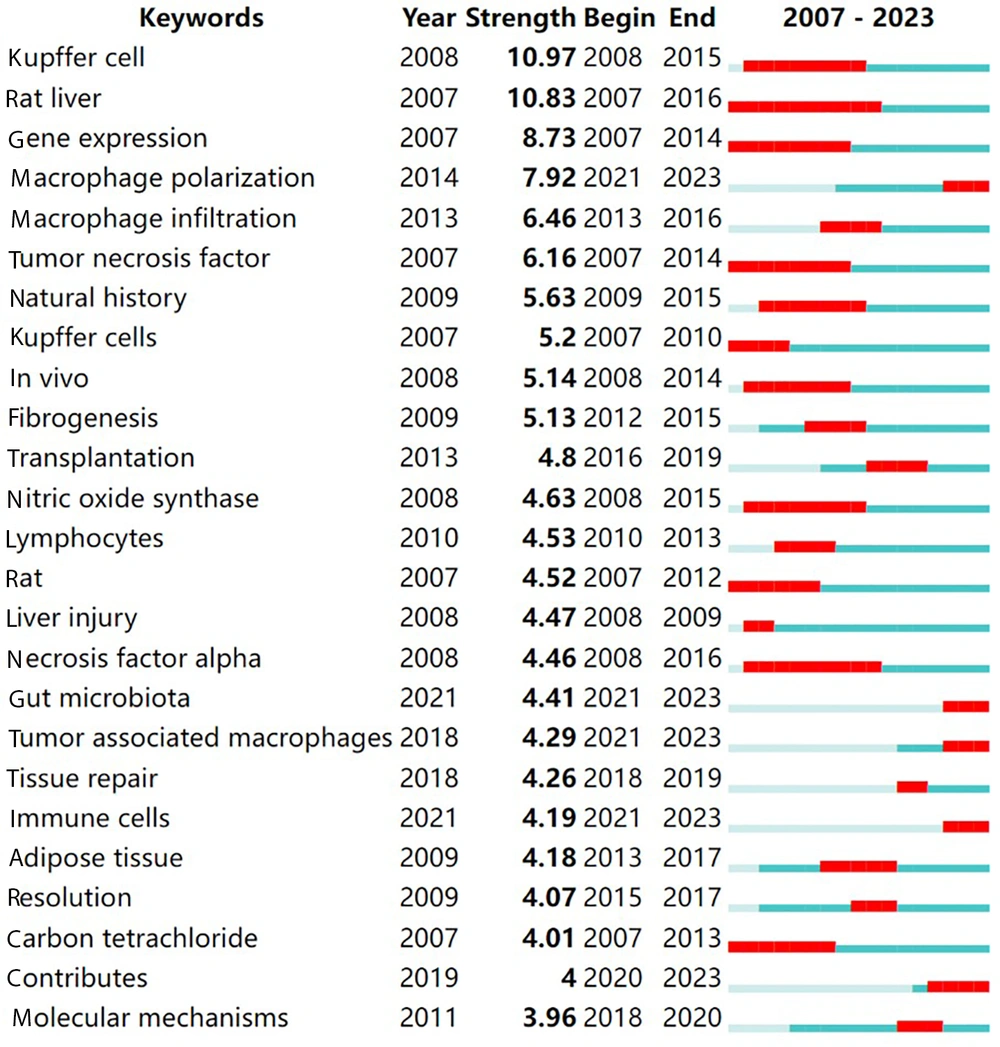
Intense citation bursts of keywords highlight the forefront of research hotspots and trends within a specific period (19). The top 25 keywords exhibiting significant citation bursts from 2007 to 2023 are displayed in Figure 5. Notably, the keywords with intense bursts include “gene expression,” “Kupffer cells,” “rat liver,” “macrophage polarization,” “macrophage infiltration,” and “tumor necrosis factor.” Keywords such as “macrophage polarization,” “gut microbiota,” “tumor-associated macrophages,” and “immune cells” emerged as recent burst keywords from 2021 to 2023, possibly indicating the latest trends in this area of research.
5. Discussion
This bibliometric analysis has examined the development of macrophage research in liver fibrosis from 2007 to the present. The analysis reveals a growing trend in publications related to liver fibrosis and macrophages. This increase mirrors a similar trend observed in a previous bibliometric study focusing on liver fibrosis treatment (20), suggesting a shift towards more experimental research rather than review articles in this field.
Apart from Italy and South Korea, all other countries within the top ten in terms of productivity also ranked in the top ten for centrality. Meanwhile, Switzerland and Austria demonstrated high centrality despite not appearing in the list of countries with the highest number of publications. This discrepancy suggests that the publication volume of the top countries may not directly correlate with their global influence. Additionally, China led in the number of publications, with significant contributions from one author, Li Jun, from the Shanghai Institute of Technology. However, China's centrality was only 0.07, and Chinese authors were absent from the list of highly cited articles, suggesting that the research quality from China in this field could be improved. This situation could be attributed to the limited number of scientific research platforms in previous years. Nevertheless, with China's growing national strength and increased investment in scientific research in recent years, its research infrastructure has seen significant improvements, leading to an increase in high-quality publications from China (21). Regarding collaboration, there was a strong linkage between the two productive countries, the USA and China, highlighting that international inter-agency collaboration and cooperation could be effectively enhanced to further advance development in this field.
The review titled “Liver Macrophages in Tissue Homeostasis and Disease” (22) by Oliver Krenkel and Frank Tacke, published in Nature Reviews Immunology, received the highest number of citations, totaling 104. Another significant review, “Mechanisms of hepatic stellate cell activation” (23) by Takuma Tsuchida, was featured in Nature Reviews Gastroenterology and Hepatology. This review focused on the activation of hepatic stellate cells as a pivotal step toward the proliferation of fibrous myofibroblasts. It explored the intricate complexity and adaptability of hepatic stellate cell activation, covering a range of processes, including autophagy, endoplasmic reticulum stress, oxidative stress, retinol and cholesterol metabolism, epigenetics, and receptor-mediated signaling pathways. It also discussed the role of extracellular signals from macrophages, hepatocytes, sinusoidal endothelial cells, natural killer cells, natural killer T cells, platelets, and B cells in the activation process. Frank Tacke authored another influential piece, “Targeting hepatic macrophages to treat liver diseases,” published in the Journal of Hepatology. This review highlighted the diversity of hepatic macrophages in terms of their ontogenesis, differentiation, and function. It emphasized the importance of understanding this heterogeneity and the distinct macrophage subsets for the critical regulation of inflammation, fibrosis, and cancer, suggesting new avenues for liver disease treatment. Additionally, Frank Tacke's publication “Macrophage Heterogeneity in Liver Injury and Fibrosis” (12) called for a deeper understanding of the mechanisms governing liver macrophage diversity and monocyte subset recruitment. It proposed that promoting restorative macrophage polarization and influencing unique macrophage effector functions could lead to innovative therapies targeting specific macrophage subsets for liver injury and fibrosis.
Inactivating stellate cells or myofibroblasts, which are pivotal in fibrosis activation, could represent a novel approach for fibrosis regression, including the induction of astrocyte apoptosis. Macrophages contribute to fibrogenesis by secreting TGFβ and other agonists. Nonetheless, they also facilitate fibrosis regression by releasing collagenase to dissolve fibrous scarring and by inducing astrocyte apoptosis and inactivating stellate cells or myofibroblasts. This dual role of macrophages, promoting fibrogenesis on the one hand and aiding fibrosis regression on the other by secreting substances like collagenase, underscores their potential as targets for fibrosis treatment (24). A thorough review of highly cited papers in this field enables a swift and comprehensive understanding of the current status and recent advancements in related research.
Regarding influential authors, Scott L. Friedman has been cited most frequently in this domain, while Frank Tacke is recognized as the most prolific author, highlighting their significant contributions to the study of macrophages and liver fibrosis. Scott L. Friedman has underlined the critical role of hepatic stellate cell activation in identifying targets for antifibrotic therapy. He also noted the importance of improving biomarkers and defining clinical trial endpoints more clearly to expedite drug approval processes (25). Furthermore, there's a pressing need for progress in validating non-invasive markers for monitoring fibrosis progression and regression, which could supplant biopsies and reduce the duration of clinical trials (26). Frank Tacke has identified soluble CD163, soluble TREM2, and sialic acid-binding immunoglobulin-like lectin-7 as potential macrophage-mediated biomarkers and has highlighted the role of macrophages in facilitating communication between different organs and compartments (27).
Keyword analysis may highlight the hotspots in this field. Liver fibrosis, hepatic stellate cells, inflammation, injury, macrophages, and Kupffer cells are all closely associated with liver fibrosis. Burst keywords like macrophage polarization, gut microbiota, tumor-associated macrophages, and immune cells indicate emerging trends in this area. We will explore these keywords further and reveal potential directions for future research as follows.
Fibrosis typically follows inflammation in nearly all cases, with the notable exception of elbow tendinosis, and involves both the innate and adaptive immune systems (28). Inflammation serves as the initial pathogenesis after liver injury, triggering monocyte/macrophage recruitment, macrophage polarization, hepatic stellate cell activation, and ultimately, liver fibrosis. Immune cells play a crucial role in regulating and balancing the fibrotic process, with T helper 2 cells and IL-4- and IL-13-activated macrophages being vital in type 2 immune responses (29).
In the experimental model of liver fibrosis, activated hepatic stellate cells and portal fibroblasts account for 90% of collagen-producing cells, highlighting their role as the primary fibroblast sources (30, 31). Hepatic stellate cells (HSCs) are activated by molecular signals such as TGF-β, Galectin-3, CCL2, and CCL5. Macrophages demonstrate considerable plasticity and can polarize into various phenotypes in response to different microenvironmental stimuli (32).
Activation of the PI3K/Akt pathway leads to the expression of anti-inflammatory cytokines, thus encouraging the development of the M2-like phenotype, which aids in tissue repair and the resolution of inflammation. On the other hand, blocking the PI3K/Akt pathway can promote the M1-like phenotype, worsening liver damage (33).
The gut microbiota has recently become a focal point of research. Bile acids play a role in the progression of liver diseases by influencing the function of gut microbiota and immune cells (34). Yang Ming and their team created a clinically relevant murine model of NASH using a typical Western-type diet, facilitating the investigation of NASH pathogenesis. Additionally, the PR119/TAK1/NF-κB/TGF-β1 signaling pathway mediates the effects of 2-oleoylglycerol on macrophage priming and the subsequent activation of hepatic stellate cells (35).
Tumor-associated macrophages can either activate or inhibit several signaling pathways in hepatocellular carcinoma (HCC) cells, including NF-κB, IL-6/STAT3, Wnt/β-catenin, TGF-β1/BMP, and ERK1/2. They achieve this by secreting cytokines and exosomes and overexpressing related proteins, thereby influencing the proliferation, invasion, and migration of cancer cells, angiogenesis, and the progression of liver fibrosis (36). Consequently, these macrophages play a role in various stages of both liver fibrosis and tumor progression (37).
Macrophages have traditionally been categorized into “pro-inflammatory” M1 and “immunoregulatory” M2 macrophages (38). Yet, this classification does not fully capture the complex roles of macrophages in both the development and resolution of liver fibrosis. In the last decade, high-resolution techniques such as single-cell RNA sequencing (scRNA-seq) (39), spatial proteomics, fate mapping experiments (40), and in vivo microscopy have greatly enhanced our understanding of macrophage origins, activation, functions, and the coexistence of pro-inflammatory and restorative macrophage phenotypes during homeostatic balance (41).
Current approaches to treating liver fibrosis include macrophage-related molecular therapies and macrophage infusion therapies (42). Nanoparticles facilitate cell-to-cell communication by carrying bioactive cargoes, such as nucleic acids, proteins, and lipids (43). Non-invasive tests, like serum-based biomarkers (44) and precision therapies involving drug and gene delivery (45), are emerging trends in this area.
5.1. Limitations
Our bibliometric analysis has some limitations. Firstly, we sourced all papers from the WoSCC, yet other databases also hold significant academic value. Secondly, our analysis was limited to documents in English, potentially excluding high-quality research published in other languages. Thirdly, we restricted our literature review to articles and reviews, excluding other document types like reports and comments, which might lead to potential omission bias. Nonetheless, despite these limitations, the integrity and reliability of our analysis remain robust, providing valuable insights for future research in this field. We hope that future studies will encompass a broader range of databases and offer a more comprehensive view of global research efforts on macrophage-mediated liver fibrosis.
5.2. Conclusions
In conclusion, research on macrophages in liver fibrosis is thriving. China is at the forefront in terms of the total number of published articles, while Germany is leading in citation frequency. This study highlighted key researchers and institutions globally involved in studies on macrophage-mediated liver fibrosis. Hepatology emerged as the most prolific journal in this research domain, receiving the highest number of citations. Importantly, studies focusing on cytokines and pathways offer promising avenues for treating liver fibrosis. Understanding how to regulate the diversity of hepatic macrophages, including the recruitment of monocyte subsets, encouraging restorative macrophage polarization, or influencing unique macrophage effector functions, could lead to novel targeted therapies for liver injury and fibrosis. Research on macrophage-based treatments is poised to become a focal point and is expected to gain increasing attention.