1. Background
Hepatitis delta virus (HDV) is a 35- 37 nm, spherical, defective virus that can cause viral hepatitis only in individuals infected with hepatitis B virus (HBV) (1, 2). It possesses an envelope composed of hepatitis B surface antigen (HBsAg), within which lies the nucleocapsid made up of HDV antigen (HDV-Ag) and RNA. The HDV is classified among satellite viruses in viral taxonomy. Delta hepatitis occurs exclusively in HBV-infected individuals because HDV does not encode its own envelope protein and utilizes HBsAg as its envelope protein for transmission (1, 2).
The HDV infection can lead to cirrhosis and liver cancer, representing the most severe forms of liver diseases caused by viral hepatitis (3). Transmission occurs through parenteral exposure in infected individuals, manifesting in two distinct forms: Coinfection and superinfection (1, 3). In coinfection, both HBV and HDV cause acute infections simultaneously, with spontaneous recovery occurring in 95% of cases. Superinfection, however, develops in a previously HBV-infected, HBsAg-positive individual and can result in fulminant hepatitis, with a chronicity rate exceeding 80%. Although less common, HDV infections can also occur through horizontal, vertical, and rare sexual transmissions (4).
It is estimated that approximately 5% of patients who have encountered HBV infection are co-infected with HDV, and coinfection is thought to account for 20% of liver diseases and liver cancers in individuals with HBV infection (5). Recent studies suggest that approximately 12 million people worldwide have encountered HDV, with a heterogeneous geographical distribution (6). It is estimated that 9 - 60 million people globally may be infected with HDV, underscoring the global significance of HDV infection. Despite recommendations for HDV screening in all HBsAg-positive patients by various international guidelines, a significant proportion of these patients remain unscreened. Consequently, estimates of HDV infection prevalence vary and may not accurately reflect the true prevalence (7). High-quality global studies are essential to ascertain the current and true prevalence of HDV infection (8).
The HDV is endemic in Mediterranean countries, northern parts of South America, Central Africa, and the Middle East (9). The HDV infection is more common in intravenous drug addicts with HBV infection in Western countries (10, 11). Turkey is located in the intermediate endemicity region concerning HDV infection. Although the prevalence of HDV infection in HBsAg-positive individuals varies by region, rates between 5 - 27% have been reported in studies (12). In some regions of the world where vaccination campaigns against HBV are carried out, the prevalence of HDV infection has also been observed to decrease significantly. Despite this, the prevalence of HDV is reported to be high in regions where HBV is endemic and in central European countries with a high immigrant population (13). Determining the prevalence of HDV in the general population, including risk groups, and estimating the relative contribution of HDV to liver disease development is critical to guide clinical care and strategies. Additionally, such studies provide information for effective public health interventions and the development of new drugs (6).
2. Objectives
This study was the first to be conducted on the Northern Cyprus population and focused on the seroprevalence of HDV (HDV-Ag and HDV-Ab) in HBsAg-positive patients in the Northern Cyprus general population.
3. Methods
3.1. Serum Samples
In our study, serum samples that were screened for HBsAg, anti-HCV, anti-HIV Ag/Ab, and syphilis in the Microbiology Laboratory of Near East University (NEU) Hospital between 2016 and 2022 were investigated. Among these, 400 serum samples of adults (aged between 18 - 84 years) residing in Northern Cyprus with only HBsAg positivity were selected randomly. Samples positive for both HBsAg and any of the anti-HCV, anti-HIV, or syphilis tests were excluded from the study. Furthermore, samples that were hemolysed, icteric, or lipemic were not used. HBsAg-positive serum samples were stored at -80°C until analysis. When they were to be used, they were removed from -80°C, allowed to reach room temperature, and then screened for HDV seropositivity (HDV-Ag and HDV-Ab). Informed consent was obtained from all patients, and this study was approved by the Near East University (NEU) Ethics Committee with the letter numbered NEU/2020/84-1172 on 22.10.2020.
3.2. Serological Analysis for HBsAg, Hepatitis Delta Virus-Ag and Hepatitis Delta Virus-Ab
HBsAg positivity of the samples was determined by using Abbott Architect chemiluminescent microparticle immunoassay (CMIA) commercial kits on the Abbott Architect automated platform. The cut-off value was 1.00 S/Co.
The presence of HDV-Ag was determined by using HDV-Ag (DIA.PRO Diagnostic Bioprobes Srl Via G., Milano-Italy) commercial ELISA (enzyme linked immunosorbent assay) kits according to the manufacturer’s instructions. Results were calculated as Cut-off = NC mean OD450 nm/620-630 nm + 0.10. The results are interpreted by the ratio of the determined cut-off value and the OD450 nm/620 - 630 nm value of the sample, resulting as cut-off/sample (Co/S). Interpretation of the results; Co/S: < 0.9 was negative; Co/S: 0.9 - 1.1 was equivocal; Co/S: > 1.1 was positive. As stated by the manufacturer, the sensitivity and specificity of the kit are above 98% (14).
The presence of HDV-Ab was determined by using HDV-Ab (DIA.PRO Diagnostic Bioprobes Srl Via G., Milano-Italy) commercial ELISA kits according to the manufacturer’s instructions. Results were calculated by means of a cut-off value determined with the formula: Cut-off = (NC+PC) / 5. The results are interpreted by the ratio of the determined cut-off value and the OD450 nm/620-630 nm value of the sample, resulting as Co/S. Interpretation of the results; Co/S: < 0.9 was negative; Co/S: 0.9 - 1.1 was equivocal; Co/S: > 1.1 was positive. As stated by the manufacturer, the sensitivity and specificity of the kit are above 98% (15).
3.3. Detection of Hepatitis Delta Virus -RNA by Real-time PCR
A commercial kit was used to extract viral RNA from 200 µL of serum (High Pure Viral RNA Extraction Kit, Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. Among various primer and probe combinations, the most effective were a pair of primers targeting the HDV antigen region, along with a TaqMan probe labeled with FAM at the 5’ end and TAMRA at the 3’ end. These components demonstrated optimal performance when used with the one-step EZ RT PCR kit from Applied Biosystems. This kit features the rTth enzyme for both reverse transcription and polymerization in a single tube, as well as UNG enzyme treatment to prevent PCR contamination in the ABI 7300 and LightCycler® 480 RT PCR System.
The forward primer (814 - 834) was 5’-GGCWCTCCCTTAGCCATCCG-3’, the reverse primer (883 - 901) was 5’-GGTCGGCATGGCATCTCCA-3’, and the TaqMan probe (850 - 871) was 5’ FAM-CTCCTWCGGATGCCCAGGTCGGAC-TAMRA-3’. The amplification product was 88 base pairs, spanning positions 814 - 901, which resides in the ribozyme region of the HDV genome.
Reverse transcription and amplification were conducted using a one-step EZ RT PCR kit in a 12.5 µL reaction mixture containing 3 mM Mn (OAc)2, 0.3 mM each of dATP, dCTP, and dGTP, 0.6 mM dUTP, 400 nM forward and reverse primers, and 200 nM TaqMan probe for both HDV and GAPDH (internal control; forward primer: TGCACCACCAACTGCTTAGC, reverse primer: GGCATGGACTGTGGTCATGAG). The mixture also included 0.01 U/µL UNG, 0.1 U/µL rTth DNA polymerase, and 2.5 µL of template RNA.
UNG was activated at 50°C for 2 minutes. Armored RNA standards were preheated at 85°C for 5 minutes. Subsequently, RNA was reverse transcribed at 60°C for 60 minutes, UNG was deactivated at 95°C for 5 minutes, followed by 40 cycles of denaturation at 95°C for 20 seconds and elongation at 60°C for 1 minute (16).
3.4. Statistical Analysis
The power analysis on the sample size was determined as α = 0.05, 1-β = 0.95 (power: 95%), the ‘sample size calculator’ was used to calculate the sample size online (17), and the suitability of the sample size of our study was confirmed.
Demographic characteristics (age, gender, nationality) of HDV-Ab positive and HDV-Ab negative patients were compared. Categorical variables are presented as number and percentage (%), and continuous variables are presented as mean with standard deviation (SD). All statistical analyses were performed using the SPSS (Statistical Package for the Social Sciences) Version 22.0 (SPSS Inc., Chicago, IL, USA) software. A Kolmogorov-Smirnov (K-S) test was used to detect the normality of the data. In the K-S test, P < 0.05 value indicates that the distribution is not normal, but P > 0.05 value indicates normal distribution of the data. Pearson chi-square test or Fisher's exact test was used to compare the categorical variables of HDV-Ab positive and HDV-Ab negative samples. Mann-Withney U test, a non-parametric test, was used to compare the means of the two groups. The values P < 0.05 were considered statistically significant.
4. Results
4.1. Characteristics of Enrolled Patients
Among the 400 HBsAg-positive patients, 293 (73.3%) were male and 107 (26.7%) were female, with a mean age of 33.1 ± 12.3 years. Regarding nationality, 49 patients (12.3%) were citizens of the Turkish Republic of Northern Cyprus (TRNC), 67 patients (16.8%) were Turkish citizens, and 284 patients (71.0%) were foreign nationals.
4.2. Hepatitis Delta Virus -Ag, Hepatitis Delta Virus -Ab, and Hepatitis Delta Virus RNA
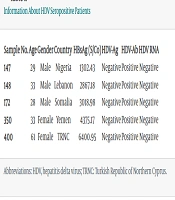
The mean HBsAg level of the patients was 3115.32 ± 1326.92 S/Co, with a range of 110.60 to 6834.03 S/Co. The anti-HDV-Ab positivity rate was determined to be 1.3% (5/400). Among male patients, the HDV-Ab positivity rate was 1.0% (3/293), while among female patients, it was 1.9% (2/107). When comparing gender and HDV-Ab positivity, no statistically significant difference was detected [odds ratio (OR) (95% CI): 1.84 (0.30 - 11.17), P = 0.404]. None of the HBsAg-positive samples contained HDV-Ag, and none of the anti-HDV-Ab-positive samples contained HDV RNA (Table 1).
| Sample No. | Age | Gender | Country | HBsAg (S/Co) | HDV-Ag | HDV-Ab | HDV RNA |
|---|---|---|---|---|---|---|---|
| 147 | 29 | Male | Nigeria | 1302.43 | Negative | Positive | Negative |
| 148 | 33 | Male | Lebanon | 2867.18 | Negative | Positive | Negative |
| 172 | 28 | Male | Somalia | 3018.98 | Negative | Positive | Negative |
| 350 | 33 | Female | Yemen | 4375.17 | Negative | Positive | Negative |
| 400 | 61 | Female | TRNC | 6400.95 | Negative | Positive | Negative |
Information About HDV Seropositive Patients
The average age of HDV-Ab positive patients was 36.80 ± 13.72 years, compared to 33.27 ± 12.97 years for HDV-Ab negative patients. There was no significant relationship between the average ages of HDV-Ab positive and negative patients (P = 0.288). Of the HBsAg-positive patients, 49/400 were TRNC citizens (locals), while the remaining patients were Turkish citizens (67/400) and foreign nationals (284/400). No significant relationship was found between nationalities and HDV-Ab positivity [OR (95% CI): 0.55 (0.06 - 5.05), P = 0.482].
5. Discussion
This study represents the first epidemiological investigation aimed at determining HDV seropositivity in the general population of Northern Cyprus. While there are reports from various countries, particularly in Europe, the literature lacks published data on HDV prevalence among HBsAg-positive patients in Northern Cyprus. In this study, HDV-Ab positivity was detected in 5 out of 400 (1.3%) HBsAg-positive patients. Although data on sex, age, and nationality were available for all patients, the low HDV positivity rate precluded analysis of potential relationships between anti-HDV positivity and patient characteristics.
Stockdale et al. (6) estimated the worldwide prevalence of anti-HDV among HBsAg-positive individuals to be 4.5%, which translates to an estimated anti-HDV positivity in the total population of 0.16%. The geographic distribution of HDV infection is heterogeneous, with particularly high prevalence reported in Western and Central Africa, Mongolia, and the Republic of Moldova (5, 6). In some parts of Europe, high rates of anti-HDV seropositivity are reported among HBsAg-positive patients. For instance, anti-HDV seropositivity in Romania, eastern Turkey, and Russia was determined to be 23% (2015), 15% (2012 - 2014), and 18 - 20% (1996 - 1998), respectively (18). A study conducted in Elazığ, Turkey, in 2019 reported anti-HDV seropositivity among HBsAg-positive individuals as 8.8% (40/455) (12). Conversely, rates in cities in western Turkey were much lower than in the east (5, 19).
Aggregate data from systematic reviews indicate that HDV is highly endemic in and around the Eastern Mediterranean Region and the Middle East (20). Another study showed that the mean prevalence of HDV was 14.7% in the Eastern Mediterranean Region (21). In our study, anti-HDV-Ab positivity was found to be 1.3% in HBsAg-positive cases, and HDV-RNA positivity was not detected in any of these samples.
The HDV infection triggers various immune responses, resulting in the generation of different markers that can be used in diagnosis. For both superinfection and coinfection, HDV-Ag and HDV RNA are biomarkers detectable in serum during the early stage of acute HDV infection (within the first ten days). However, HDV-Ag is transient and may disappear shortly thereafter. Due to this characteristic, HDV-Ag is not reliable as a diagnostic marker; nonetheless, a positive HDV-Ag still indicates active infection (22). At the end of the acute phase in coinfection, levels of most HDV biomarkers are reduced, indicating viral clearance (23). In contrast, superinfection often leads to chronicity, with persistent antibody levels and HDV RNA positivity (8).
Most antibody tests used are ELISA kits; however, the accuracy of both internal and commercial quantitative evaluations of HDV RNA can vary significantly, and HDV RNA may occasionally be undetectable in samples that are actually HDV RNA positive. It is important to consider the secondary structure and various genotypes of HDV when developing primers and probes (24). Additionally, the selection of quality control items impacts the precise quantification of HDV RNA. Quality control products for viral nucleic acids should ideally assess the detection process quality and serve as a basis for evaluating procedures and comparing results across different laboratories (25). Furthermore, HDV RNA tests become negative when the virus has been cleared, either spontaneously or through treatment (26).
In our study, HDV-Ag and HDV RNA were not found positive in any patient sample with HDV-Ab positivity. Therefore, we believe that none of the patients were in the acute infection period when the samples were collected, and HDV-Ab positivity was due to a past infection. Recently, a decrease in HDV prevalence has been reported in many European regions, particularly in Southern European countries, due to effective vaccination programs against HBV, compulsory screening tests for blood donors, improved hygiene conditions, and behavioral changes (27). Conversely, it has been reported that prevalence, especially in France and the United Kingdom, has increased due to migration from regions where HDV is endemic and HBV vaccination is uncommon (28-31).
The HDV prevalence and genotype distribution vary greatly across different countries and regions (32). However, since studies conducted worldwide generally focus on risk groups (e.g., intravenous drug users, individuals exhibiting high-risk sexual behavior, patients with human immunodeficiency virus (HIV) or hepatitis C virus (HCV) infection), the actual HDV prevalence remains unknown (33). No previous research on HDV seroprevalence has been conducted in Northern Cyprus. Therefore, we lack information about HDV dynamics in the country.
Cyprus is the third largest island in the Mediterranean, with two distinct communities residing on the island. The northern side, known as the Turkish Republic of Northern Cyprus (TRNC), is predominantly populated by Turkish Cypriots, while the southern side, the Republic of Cyprus, is mainly inhabited by Greek Cypriots (34). According to the literature, the prevalence of HBV infection in TRNC is considered to be of low endemicity (35-37). Our results assess the impact of HDV infection in HBV-positive individuals in Northern Cyprus, highlighting the risk of HDV coinfection or superinfection. This study underscores the importance of determining the HDV burden in Northern Cyprus and globally. Due to the HDV burden, increased efforts are needed to prevent or eliminate the rapid and severe progression of liver diseases through screening, prevention, and treatment.
Currently, there is no data on HDV prevalence in Northern Cyprus. Based on the data obtained from our study, we can infer that the rate of HDV seropositivity in Northern Cyprus is low. The true prevalence may be revealed through future studies conducted among high-risk groups, such as drug users and individuals exhibiting high-risk sexual behavior (HRSB). The patient samples included in our study were derived from the general population and individuals who have obtained work permits in Northern Cyprus. Additionally, we believe there is insufficient molecular profile data on viral hepatitis in such specific groups or the general population.
There were several limitations to this study. As a retrospective cross-sectional study, the patient serum samples might have been obtained at different stages of HDV infection. Furthermore, the number of patients within stratified groups was often too low to determine the significance of findings among groups.