1. Background
Liver tumors are relatively common, with a prevalence of about 9%. Fortunately, most are benign, asymptomatic, and incidentally identified through imaging. The three most common types of benign liver tumors include hemangioma, focal nodular hyperplasia, and hepatocellular adenoma (HCA) (1).
Hepatocellular adenomas are known to be benign, solid, and rare hepatocellular tumors. According to previous studies, approximately 3 - 4 cases of HCA per 100,000 population are prevalent in North America and Europe, with slightly lower prevalence in Asian countries. The highest prevalence has been reported in women who use oral contraceptives (OCPs) and are aged 20 to 40 years. However, other risk factors may increase the likelihood of developing HCA, including the use of anabolic steroids, obesity and metabolic syndromes, hereditary syndromes such as glycogenosis type 1, MODY type 3 (maturity onset diabetes of the young), familial adenomatous polyposis, and anemia, including Fanconi anemia (2).
The tumor is often found following abdominal pain or incidentally on imaging and is rarely manifested by bleeding or malignant changes. Although histological analysis is the typical method for diagnosing HCA, even experienced pathologists may have difficulty distinguishing it from focal nodular hyperplasia and hepatocellular carcinoma (HCC) (3).
Based on molecular classification, HCAs have been recently categorized into six major subgroups: Adenomas inactivated for HNF1A, inflammatory adenomas, β-catenin-activated adenomas mutated in exon 3, β-catenin-activated adenomas mutated in exon 7 - 8, sonic hedgehog adenomas, and unclassified adenomas (4). Classification of HCA subtypes based on imaging, molecular, and immunohistochemical markers is very helpful in predicting the risk of HCAs progressing to HCC and can lead to the choice of the best therapeutic approach for patients (5). The classification of HCA subtypes using immunohistochemistry is completely valid (6).
1.1. HNF1A-mutated Hepatocellular Adenoma
This subtype comprises 35 - 40% of HCAs (7). Mutations occur in HNF1α and are associated with steatosis and decreased expression of liver fatty acid-binding protein (LFABP) (8).
1.2. Inflammatory Hepatocellular Adenoma
This is relatively common, accounting for about 40 to 50% of all HCAs. Unlimited activation of the IL6/JAK/STAT inflammatory pathway characterizes this type of HCA. Due to the activation of inflammatory pathways, an increase in serum amyloid A (SAA) and C-reactive protein (CRP) expression is observed in the cytoplasm. Sinusoid dilatation, inflammation, and ductular reaction are identified in this group (8).
1.3. β-catenin-Activated Hepatocellular Adenoma
This subtype includes 10-15% of HCAs (7). The CTNNB1 gene is responsible for encoding β-catenin, a crucial molecule in the Wnt signaling pathway. Once β-catenin is transported to the nucleus, it triggers the transcription of various genes involved in hepatocyte physiology, including those related to cell proliferation, stem cell renewal, epithelial-mesenchymal transition, and cell adhesion. Mutations in the β-catenin gene are found in 15 - 30% of HCAs. The HCAs with the β-catenin mutation have a higher likelihood of progressing to HCC (up to 50%) (9). Activating mutations in the β-catenin gene are associated with a high risk of HCC and are detectable by high cytoplasmic staining with glutamine synthetase (GS) and nuclear staining with β-catenin (8).
1.4. Unclassified Hepatocellular Adenoma
This subtype includes 5 - 10% of HCAs (3). Cases are categorized in this group when pathological findings and immunohistochemical stainings do not match any of the above groups (8). For this group, the genotype and phenotype are unknown, and immunohistochemistry results are nonspecific. Additionally, HCAs that cannot be classified due to near-total necrosis or hemorrhage are placed in this group (10).
1.5. Sonic Hedgehog Hepatocellular Adenoma
An additional subtype recently reported involves the activation of the sonic hedgehog pathway, leading to the expression of prostaglandin D. In this subtype, the risk of hemorrhage increases even in small nodules (11).
Given the importance of identifying HCA subtypes in predicting the risk of their progression to malignancy and bleeding, it is necessary to classify them correctly. The risk of converting β-catenin HCA to HCC is greater than that of other subtypes. Conversely, some subtypes (even those larger than 5 cm) can be conservatively followed up. Nevertheless, the management of HCAs remains a topic of controversy. The decision to pursue conservative or surgical treatment options ultimately depends on the specific clinical context. However, molecular analyses of HCAs have significantly contributed by identifying various molecular characteristics that can predict the risk of malignant transformation.
Based on the latest molecular data and existing clinical features, surgical resection of HCA is recommended if: (A) The HCA is large (> 5 cm) with an imminent risk of rupture or hemorrhage; (B) there is evidence of β-catenin activation in any HCA; (C) the HCA is present in male patients; (D) dysplasia or atypia is detected within an HCA; (E) clinical features suggest malignant transformation in HCA, such as increasing size and malignant imaging characteristics (9).
2. Objectives
This study aims to determine the frequency of different subtypes of HCA using immunohistochemistry in resected material and needle biopsies in our hospital, as well as the correlation of subtypes with morphologic findings, diagnostic images, and clinical parameters.
3. Methods
This cross-sectional study was conducted on all resection specimens and needle biopsies received in the pathology department of Imam Khomeini Hospital Complex (IKHC) over the past ten years. Initially, samples were collected from 28 patients, but eight patients were excluded based on our exclusion criteria (see below). Ultimately, 20 patients with HCA were enrolled.
3.1. Inclusion Criteria
We included HCA samples obtained from needle biopsies and resection materials of patients admitted to IKHC over the past ten years, with paraffin blocks available in the hospital's pathology department.
3.2. Exclusion Criteria
- Inadequate residual tumor in paraffin blocks for immunohistochemical studies.
- Poor quality of paraffin blocks.
- Incomplete clinical information.
- Technical problems with immunohistochemistry.
Initially, the clinical information of all patients was retrieved from the hospital information system (HIS) from 2017 to 2020. Older documents not available in HIS were searched using Microsoft Word files present in the archives. Subsequently, we gathered the paraffin blocks of needle biopsies and resection materials from the pathology department of IKHC. Simultaneously, we collected demographic and medical data from patients, including age, sex, Body Mass Index (BMI), use of OCPs and androgens, lesion number and size, other systemic diseases, and patient symptoms at the time of referral, from patient charts or direct inquiry via telephone call.
Needle biopsies and resected specimens were reviewed by two pathologists, and subtypes of HCAs were determined based on morphology. Immunostainings for LFABP, β-catenin, CRP, SAA, glypican-3, and GS markers were then performed. The results of the classification of HCAs by immunohistochemistry were compared with the initial morphologic subtype categorization and radiologic classification using MR imaging. Additionally, clinical features were evaluated in each immunohistochemical subtype.
For immunohistochemical subclassification into H-HCA, IHCA, β-HCA, and UHCA, the following criteria were utilized:
- H-HCA: Negative immune-reaction for LFABP staining.
- IHCA: Positive cytoplasmic immune-reaction for SAA or CRP staining.
- β-HCA: Diffuse and strong cytoplasmic staining with GS and/or positive nuclear immune-reaction with β-catenin (12).
- U-HCA: The absence of the above-mentioned findings.
According to Margolskee et al's study, GS staining results were categorized as follows:
- 0: Negative or weak perivenular staining in less than 10% of tumor cells.
- Score +1: Perivascular staining in more than 10% of tumor cells.
- Score +2: Strong diffuse staining.
SAA, CRP, and LFABP stainings were scored as:
- 0: Negative or less than 10% of tumor cells showing staining.
- Score +1: Between 10 - 50% of tumor cells showing staining.
- Score +2: More than 50% of tumor cells showing staining (13).
According to this study, HCAs with GS, SAA, CRP, and LFABP staining in less than 10% of tumor cells were considered negative.
The antibodies used were as follows:
- Anti-liver FAP antibody (L2B10, monoclonal mouse antibody) from Abcam, diluted to 1/50.
- Anti-CRP antibody (Y284, monoclonal rabbit antibody) from Abcam, diluted to 1/200.
- Anti-SAA antibody (115, monoclonal mouse antibody) from Abcam, diluted to 1/500.
- Anti-GS (3B6-BSA and azide free, monoclonal mouse antibody) from Abcam, diluted to 1/400.
- β-catenin (EP35, rabbit anti-human β-catenin monoclonal antibody) from Master Diagnostica, ready-to-use.
- Anti-glypican-3 (BMS059, mouse antibody) from Zytomed System, ready-to-use.
The antigen retrieval method was performed using heating and microwave. Finally, statistical analysis of the data was conducted using version 16 of SPSS software, utilizing tests such as chi-square.
4. Results
In this study, 20 cases of HCAs were included, consisting of 18 females (90%) and two males (10%). The ages of patients ranged from 18 to 52 years, with a mean age of 35.7 years (± SD 9.2). According to immunohistochemical findings, HCAs were classified as inflammatory in 11 patients (55%), steatotic adenomas in four patients (20%), β-catenin type in two patients (10%), and unclassified type in three patients (15%). Typical histologic findings and immunostaining results of inflammatory, steatotic, and β-catenin HCAs are shown in Figures 1 - 3. Patient information is included in Table 1. Clinical information and radiologic imaging were unavailable for two women.
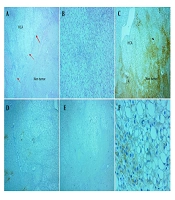
A and B, Histologic features of inflammatory adenoma revealed sinusoidal dilatation and inflammation (H&E, 40x, 100x, respectively); C, immunohistochemical staining with glutamine synthetase (GS) was negative (IHC, 400x); D, liver fatty acid-binding protein (LFABP) was diffusely expressed (IHC, 100x); E, CRP was diffusely positive (IHC, 100x); F, serum amyloid A stain showed negative result (IHC, 100x).
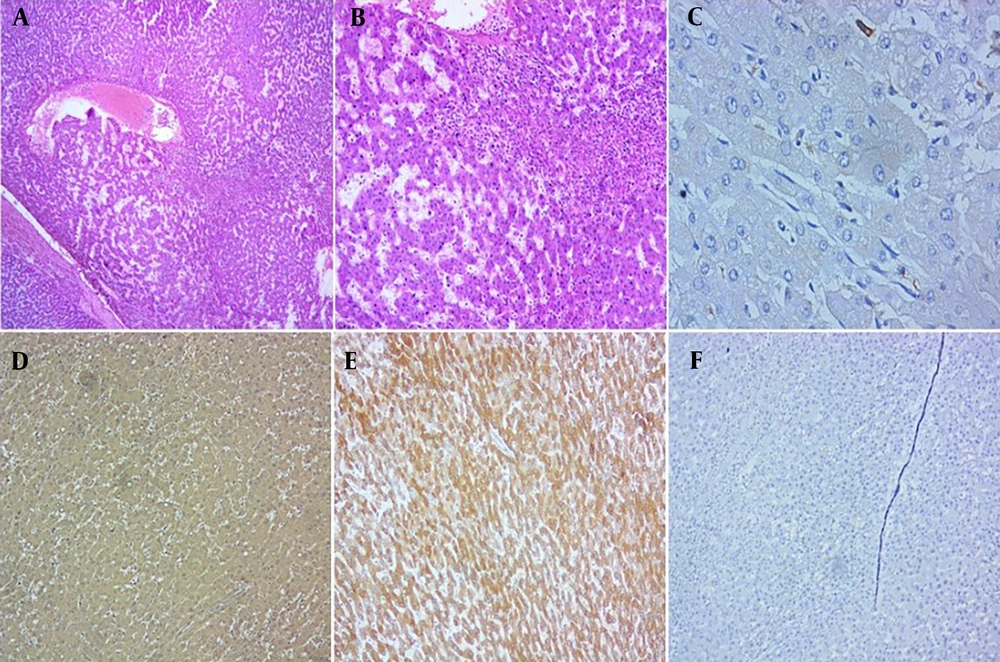
A and B, Histologic features of steatotic adenoma (H&E, 40x, 100x, respectively); C, immunohistochemical staining with liver fatty acid-binding protein (LFABP) revealed loss of expression in tumor cells (IHC, 400x); D, glutamine synthetase (GS) showed negative result (perivascular expression in peripheral liver tissue and less than 10 percent in adenoma, IHC, 40x); E, C-reactive protein (CRP) stain demonstrated negative result (IHC, 40x); F, β-catenin stain revealed membranous pattern without nuclear staining (IHC, 400x).
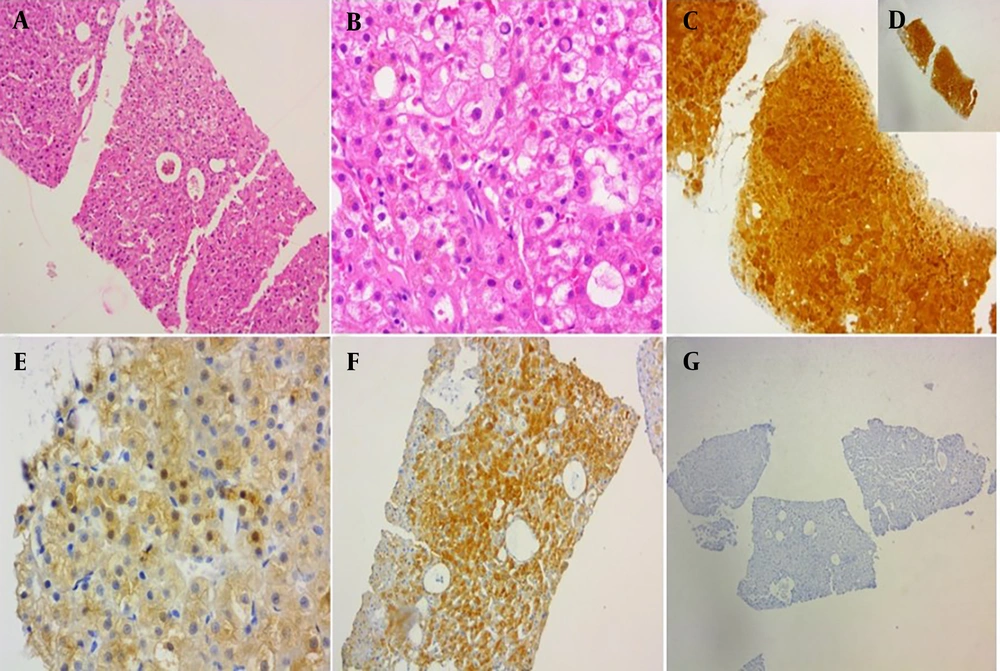
A and B, Histologic features of β-catenin adenoma revealed pseudoglandular formation and mild cytologic atypia (H&E, 100x, 400x); C and D, glutamine synthetase (GS) showed strong and homogeneous staining (IHC, 400, 100x); E, β-catenin stained some nuclei (IHC, 400x); F, liver fatty acid-binding protein (LFABP) revealed positive result (IHC, 100x); G, serum amyloid A stain showed negative result (IHC, 100x).
| Characteristics | H-HCA | IHCA | β-HCA | Unclassified |
|---|---|---|---|---|
| Number of cases (No. %) | 4 (20) | 11 (55) | 2 (10) | 3 (15) |
| Mean age (y) | 36.25 | 35.09 | 34.5 | 38 |
| Mean BMI (kg/m2) | 24.85 | 28.6 | 31.4 | 31.5 |
| Gender | ||||
| Female | 4 | 10 | 1 | 3 |
| Male | 0 | 1 | 1 | 0 |
| Symptoms | ||||
| Abdominal pain | 2 | 7 | 1 | 1 |
| Non-specific | 0 | 2 | 1 | 0 |
| Asymptomatic | 1 | 2 | 1 | 0 |
| OCP/androgen use | ||||
| Yes | 1 | 10 | 2 | 1 |
| No | 2 | 1 | 0 | 1 |
| Size of lesions (cm) | ||||
| ≤ 5 | 2 | 5 | 0 | 1 |
| > 5 | 1 | 5 | 2 | 0 |
| Number of adenomas | ||||
| Solitary | 2 | 9 | 1 | 1 |
| Multiple | 2 | 2 | 1 | 2 |
Clinical Characteristics of Hepatocellular Adenoma Subtypes
Fourteen patients (77%) had a history of OCP or androgen use, including 13 females (81% of females) and one male (50% of males). The different microscopic subtypes in patients taking OCPs or androgenic medications are shown in Table 1. Both patients in the β-catenin subgroup had a history of OCP/androgen intake (100%), and 90.9% of the patients consumed OCPs in the inflammatory subtype. This rate was 50% in the unclassified group and 33% in the steatotic group. However, these differences were not statistically significant (P = 0.11). The number of HCAs, the presence of underlying diseases, and higher BMI were not statistically correlated with the subtype of HCA (P = 0.35, 0.50, and 0.42, respectively).
MR imaging of 16 patients was available and reviewed by the radiologist. Of these cases, seven patients (45%) were classified as IHCA, four patients (25%) as β-catenin type, three patients (18%) as steatotic group, and two patients (12%) as unclassified subtype. These tumors were radiologically divided into two groups according to size: (A) Smaller than 5 cm and (B) larger than 5 cm; eight patients (50%) were below 5 cm and eight patients (50%) were over 5 cm. Among patients with inflammatory adenomas, 50% were below 5 cm and 50% over 5 cm; among patients with steatotic adenoma, 66% were over 5 cm and 33% below 5 cm; 100% of the β-catenin subtypes were over 5 cm in size, and the unclassified group were 100% below 5 cm (Table 1). Pathologic and radiologic diagnoses were concordant in 12 patients (75%). The steatotic and β-catenin subtypes showed better radio-pathological agreement.
5. Discussion
In the present study, we analyzed the prevalence of various types of liver cell adenomas by immunohistochemistry in 14 resected adenomas (70%) and 6 needle biopsies (30%). The patients’ ages ranged from 18 to 52 years, with 90% of the cases being female, and 14 subjects (77%) having a history of OCP/androgen consumption. The average BMI was in the overweight range (28.5 kg/m2).
Initially, we classified adenomas based on morphology on H&E sections into four groups as follows: IHCA was identified by sinusoidal dilatation, inflammation, and ductular proliferation. H-HCA was characterized by steatosis. In β-catenin adenoma, there was cytologic atypia and pseudorosette formations. If the above-mentioned features were absent, the cases were considered unclassified.
We classified 12 HCAs (60%) as the inflammatory type, 5 HCAs (25%) as the steatotic type, 1 HCA (5%) as the β-catenin type, and two HCAs (10%) as unclassified type based on morphologic findings. The most common adenoma subtype was the inflammatory type, followed by the steatotic, unclassified, and β-catenin types, respectively.
We then applied immunostaining with the following final classifications: Eleven IHCAs (55%), four steatotic HCAs (20%), two β-catenin HCAs (10%), and three unclassified HCAs (15%). Adenomas could be correctly classified by morphology alone in 13 cases (65%).
Considering morphologic findings, one case was classified as the inflammatory type due to marked sinusoidal dilatation, but immunostaining showed diffuse and strong GS staining, leading to its reclassification as the β-catenin type. Additionally, two adenomas were morphologically placed into the steatotic type due to some degree of steatosis, but immunostainings confirmed their inflammatory nature.
Although steatosis and sinusoidal dilatation are considered morphologic hallmarks of H-HCA and IHCA, respectively, these findings are not reliable and can be identified in other types of adenoma. Bioulac-Sage et al.’s study identified the presence of steatosis in 95.6% of adenoma cases, and even some cases of IHCA and β-HCA showed severe steatosis (more than 60%). Additionally, in this study, sinusoidal dilatation was present in 11 H-HCAs, nine β-IHCAs, and three U-HCAs out of a total of 114 cases. These findings demonstrate that morphologic diagnosis alone may not determine adenoma subtypes correctly, and using confirmatory tests, including immunohistochemistry, in the classification of adenomas is necessary (14).
The HCAs with a change in subtype classification after applying immunostaining are listed in Table 2. As mentioned, the most common subtype of adenoma in our study was the inflammatory type, consistent with most previous studies (15, 16). A study from Shiraz, Iran, assessed 40 patients with HCAs over 10 years and subclassified them by immunohistochemical staining. In this study, H-HCA was reported as the most common subtype of adenoma (50% H-HCA) (17). One reason for this discrepancy may be the higher ratio of resection to total specimens in our study compared to Geramizadeh et al.’s study (i.e., our study included 14 resections and 6 biopsies, whereas the Geramizadeh et al.’s study comprised 15 resections and 25 biopsies). Patients with IHCA are more likely to be symptomatic (e.g., acute abdominal pain or hemorrhage) and undergo surgery, leading to a higher frequency of IHCA in our study. Another reason may be the higher incidence of OCP usage in our study.
| Morphologic Initial Classification of HCA | IHC Final Classification of HCA |
|---|---|
| Steatotic HCA | Inflammatory HCA |
| Inflammatory HCA | Unclassified HCA |
| Steatotic HCA | Inflammatory HCA |
| Inflammatory HCA | β-catenin HCA |
| Inflammatory HCA | Unclassified HCA |
| Unclassified HCA | Inflammatory HCA |
| Inflammatory HCA | Steatotic HCA |
Hepatocellular Adenomas with Change in Subtype Classification After Immunostaining
Bellamy et al.’s study which was conducted in United Kingdom, reported the incidence of adenomas as follows: 23.4% were inflammatory, 7.8% β-IHCA, 11.1% β-HCA, and 30.6% non-classified. Additionally, inflammatory adenomas were associated with metabolic syndrome and alcohol consumption (18). In the United States, Shafizadeh et al. reported a different incidence of HCA in 28 patients. None of the patients were in the β-catenin group, only one patient was in the steatotic group (3.6%), 9 patients had inflammatory adenomas (32.1%), and the rest were in the non-categorical group (3). Studies from Japan (Sasaki and Nakanuma) and France (Bioulac-Sage et al.) reported the incidence of IHCA as 39% and 39.8%, respectively, and H-HCA as 15% and 10.8%, respectively (16, 19). Although the results of these two studies differ somewhat from ours, the most common subtype of adenoma in both studies is the inflammatory type, similar to our study.
In our study, abdominal pain was the most common clinical symptom. While HCAs have been asymptomatic in most other studies, this discrepancy may be related to the higher number of resection specimens in our study.
There was no significant relationship between age, BMI, adenoma number, underlying disease, and adenoma phenotypic group. Although the history of contraceptive/androgen usage was greater in the inflammatory and β-catenin groups, the difference did not reach statistical significance in our study, likely due to the limited sample size. The main risk factor for developing HCA is estrogen or androgen usage (14). In our study, a history of OCP consumption was identified in 13 out of 16 women (information on OCP usage, BMI, and metabolic syndrome was not available for 2 women). Nevertheless, about 18% of women (3 patients) were not exposed to OCPs. All women with IHCA had a history of OCP usage. The high consumption of OCPs, overweight (average BMI in the overweight range: 28.5 kg/m2), and metabolic syndromes are correlated with a high frequency of IHCA in our study.
Although OCP usage is more commonly associated with IHCA, it should be noted that other subtypes of adenoma can also have a history of OCP consumption. For example, in our study, one case of H-HCA and one case of U-HCA had a history of OCP consumption. In some studies, SAA is reported as a good marker for detecting IHCA with high sensitivity and specificity, whereas CRP is less specific, especially in differential diagnosis with focal nodular hyperplasia (19, 20). However, a review article in 2012 reported that the sensitivity and specificity for CRP were more pronounced than SAA (12). In our study, in two cases of IHCA, only one marker (SAA or CRP) was expressed. Therefore, for the diagnosis of this type of adenoma, it is best to use both SAA and CRP.
Because the risk of converting β-catenin HCA to HCC is greater than others, surgical management of patients with HCA, in addition to male gender, tumor size (> 5 cm), and androgen usage, depends on the presence or absence of a β-catenin mutation (9). The present study had two cases (10%) of β-HCA. The frequency of β-catenin mutation is reported to be about 10 - 15%, in agreement with the present study (4, 15).
In this regard, one case was associated with Fanconi anemia. The patient was an 18-year-old man treated with androgen (danazol), presenting with an incidental hepatic mass on imaging. Microscopic examination showed mild cytologic atypia and focal pseudoglandular formation. Immunostaining revealed diffuse and strong GS staining, focal nuclear positivity with β-catenin, and a negative result for glypican-3 (Figure 3). In Fanconi anemia, there is an increased risk of several tumors. In these patients, androgen treatment and iron overload may lead to the development of HCA and carcinoma (21).
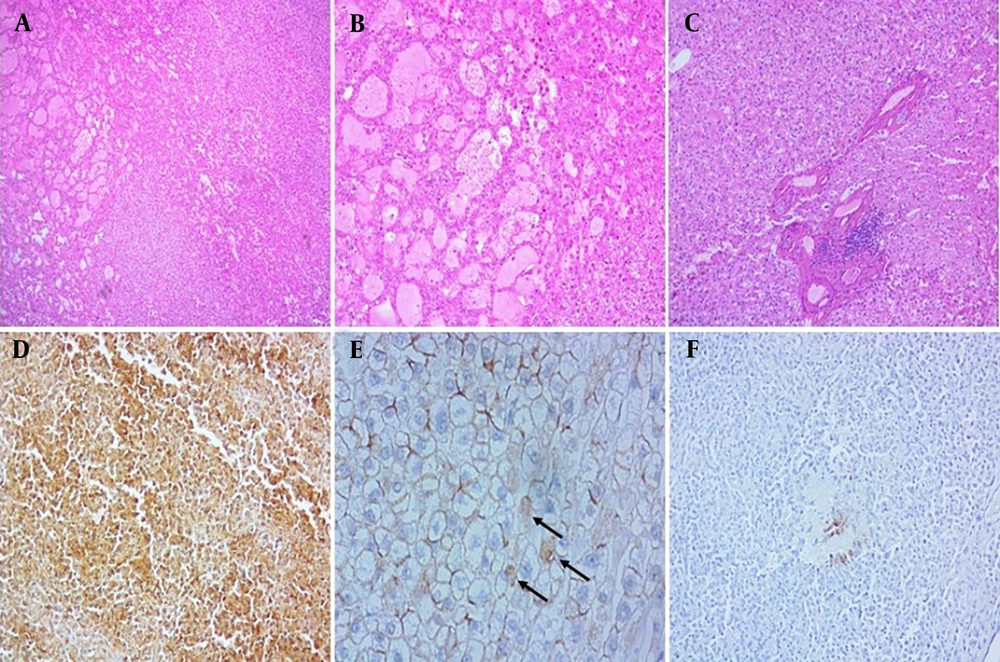
Another case involved a 51-year-old woman with a prior history of OCP consumption who underwent surgery. Microscopic examination revealed marked dilatation of sinusoids accompanied by inflammation, favoring inflammatory adenoma. Both pathologists reported it as inflammatory adenoma based on morphologic findings; however, immunohistochemistry showed diffuse, strong, and homogeneous GS staining, with β-catenin staining a few nuclei. The CRP and SAA stains were negative (Figure 4). According to WHO classification (11), this type is the β-catenin-mutated HCA Exon 3, which is at high risk for progression to HCC. The absence of cytologic/structural atypia, such as pseudoglandular formation, in this case highlights the importance of immunostaining for definite typing of HCAs, particularly in needle biopsies.
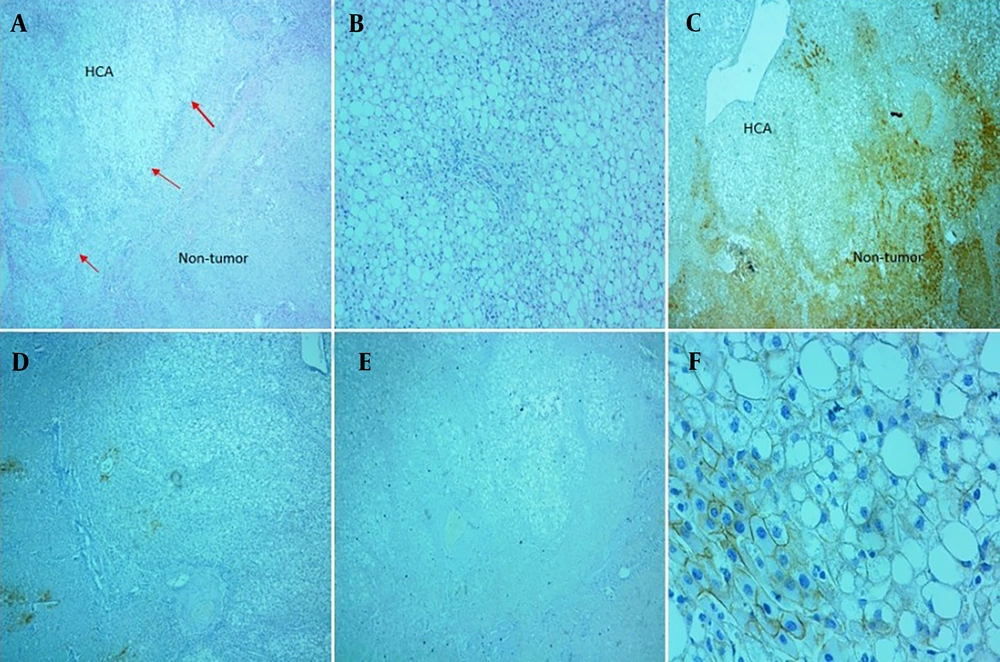
A, B and C, Histologic features of β-catenin-mutated Exon 3 adenoma showed marked dilatation of sinusoids accompanied by inflammation, without significant cytological atypia (H&E, 40x, 100x and 400x, respectively); D, immunohistochemical staining with glutamine synthetase (GS) was diffusely positive (IHC,100x); E, β-catenin was expressed in few nuclear hepatocytes, (arrows), (IHC, 400x); F, serum amyloid A showed negative result (expression in less than 10 percent in tumor cells), (IHC, 100x).
In our study, sonic hedgehog HCA was not investigated because the prostaglandin D marker was unavailable. Additionally, some cases of unclassified HCA may have been included in this group, which is important due to the increased risk of hemorrhage. This inclusion is a limitation of our study. For determining the β-catenin subtype, a clear interpretation of GS staining is essential. However, there are instances where GS staining results may be equivocal, and in such cases, molecular studies play a crucial role in classification (11). This highlights a limitation of relying solely on immunohistochemical staining for the definitive categorization of adenomas.
Additionally, the staining of these markers in HCC is important. For example, in a study by Liu et al., SAA was positive in 17% of HCCs, CRP was positive in 54% of HCCs, and loss of LFABP was observed in 23% of HCCs. These data reveal that immunostaining for subtyping of hepatic adenoma is not useful for differentiating between hepatic adenoma and HCC (22).
5.1. Conclusions
Definite classification of HCA subtypes, particularly in needle biopsies, is critical, as it is one of the most important factors in clinical decision-making and surgical management. Our findings indicate that histologic findings alone cannot accurately determine adenoma subtypes in all cases. Therefore, the use of immunohistochemistry or molecular analysis in the classification of adenomas is necessary.