1. Background
Hepatitis C virus (HCV) and hepatitis B virus (HBV) are bloodborne pathogens that progress through acute and chronic phases, leading to severe liver conditions such as hepatic steatosis, liver fibrosis, cirrhosis, hepatocellular carcinoma (HCC), and end-stage liver disease (1-4). Globally, HBV infects an estimated 5% of the population, while HCV affects about 3% (5). Persistent infections result in chronic hepatitis in 60 to 80% of cases. As of 2022, the World Health Organization reported 58 million cases of chronic HCV infection and 257 million cases of chronic HBV infection (3-5).
Persistent viral activity and intermittent hepatitis flares contribute to ongoing hepatocellular damage, leading to disease progression and liver fibrosis in both chronic hepatitis C (CHC) and B (CHB) infections. Viral proteins disrupt lipid metabolism, modify signal transduction in infected hepatocytes, induce the production of oxygen radicals and profibrogenic mediators, particularly TGF-β1, and promote inflammation by releasing C-C motif chemokine ligand 2 (CCL2). These processes trigger the transformation of hepatic stellate cells (HSCs) into myofibroblasts, which produce collagen. Myofibroblasts play a crucial role in liver fibrosis progression and are the primary focus of antifibrotic interventions. Accurate liver fibrosis staging is essential for managing patients with hepatitis C or B infections (6-8).
Liver biopsy presents limitations due to invasiveness and contraindications. The METAVIR score, a key tool for assessing necroinflammatory activity and liver fibrosis in CHC and CHB patients, utilizes fibrosis and activity scores derived from liver biopsy samples. Non-invasive methods, such as ultrasound elastography for liver stiffness and identification of fibrosis markers, offer valuable alternatives (9-11).
Authors, through multicenter studies, confirm the effectiveness of multiple non-invasive serum parameters in distinguishing mild and severe liver fibrosis in CHC and CHB. The integration of non-invasive fibrosis parameters into routine practice gained momentum with the advent of direct-acting antiviral (DAA) therapy for HCV infection, offering rapid and effective patient healing and paving the way for HCV eradication. In the current era of DAA therapy, although global implementation faced socioeconomic limitations, treatment policies now prioritize vulnerable patients. Recognizing the simplicity, accessibility, and cost-effectiveness of serum non-invasive parameters, they have become vital for categorizing advanced fibrosis cases. With well-defined DAA protocols, where fibrosis degree no longer dictates treatment decisions, non-invasive markers are crucial in monitoring post-therapy outcomes for chronic hepatitis C patients. The role of serum markers, both in patients with CHC or CHB, extends to identifying individuals at risk of HCC and underlying liver diseases sustaining fibrogenesis, enabling early planning of diagnostic procedures and HCC surveillance (12-15).
The AST/ALT ratio, APRI Score, FIB-4 Score, and Forns index are the most commonly used serum markers for assessing liver fibrosis (16-18). Notably, the FIB-4 Score is widely accepted as the most reliable marker for demarcating advanced liver disease (19).
2. Objectives
The study aimed to investigate the feasibility of utilizing various serum markers to assess fibrosis severity in patients with both HBV and HCV infections, taking into account the different etiopathogenesis of the resulting fibrosis. Hypothetically, we hypothesize that certain serum markers can be relied upon to assess advanced liver fibrosis, regardless of whether it is caused by chronic HCV or HBV infection, while consideration of the etiology of chronic viral hepatitis is necessary for others.
3. Methods
This cross-sectional retrospective single-center study included 389 patients diagnosed with chronic hepatitis C and B at the Clinic for Infectious Diseases, University Clinical Centre of Vojvodina, from January 2021 to September 2023. Diagnoses and liver lesions were determined through patient history, clinical examination, laboratory testing, ultrasound elastography, and liver biopsy, followed by sample interpretation by a single specialist in pathology, an expert in liver diseases. Inclusion criteria encompassed an age range of 18 to 75 years, a confirmed diagnosis of CHC or CHB, and written consent. Exclusion criteria considered factors potentially influencing studied parameters, excluding patients with liver carcinoma, alcoholic, cholestatic, or autoimmune liver disorders, prior antiviral or immunosuppressive medication use, dementia, psychiatric disorders, or intravenous drug abuse within six months. Ultimately, data analysis included 327 patients meeting the specified criteria, and collected information covered demographic details, laboratory parameters, and liver biopsy results using the METAVIR score.
All baseline laboratory tests relevant to this study were completed within 24 hours of admission. Key serological measurements for this investigation comprised liver function tests, lipid profiles, and complete blood counts.
Evaluation of hepatic fibrosis employed various criteria: APRI score, AST/ALT ratio, Fibrosis 4 index (FIB-4), Forns index, and the METAVIR scoring system. The METAVIR score uses two measurements made from the appearance of a sample obtained from a liver biopsy—the fibrosis score and the activity score. The fibrosis score is used to describe the amount of inflammation in the liver: (1) F0: No fibrosis; (2) F1: Portal fibrosis without septa; (3) F2: Portal fibrosis with few septa; (4) F3: Numerous septa without cirrhosis; (5) F4: Cirrhosis. F3 and F4 METAVIR scores suggest advanced fibrosis (9, 10).
Among the 327 patients, 274 with CHC underwent pathohistologically confirmed liver fibrosis assessment through biopsy (cohort A), while 53 with CHB constituted group B.
Data analysis involved creating a database in Excel and transferring it to SPSS v 23.0 for statistical analysis. Categorical data were presented using whole numbers and percentages, and numerical continuous variables were described with mean values and standard deviation or median with interquartile range. Normality of distribution and equality of variances were assessed, and differences in continuous variables between groups were tested using t-test or Mann-Whitney U test. Spearman’s correlations examined the association between indexes and the grade of fibrosis.
Receiver operator characteristic (ROC) analysis with area under the curve (AUC) calculation was employed to test and compare the capabilities of indexes in predicting advanced fibrosis.
4. Results
The study involved 327 patients, with the majority (83.8%, 274/327) diagnosed with CHC. The population was predominantly male (66%, 215/327), with a mean age of 42 years. Women were older than men (45.5 vs. 36.0, P < 0.001). Calculated scores (Forns, APRI, FIB-4) showed no significant gender differences, except for higher AST/ALT in females (0.75 vs. 0.61, P < 0.001). Table 1 details baseline characteristics of CHC and CHB groups. CHC had a higher male prevalence (86.8% vs. 61.6%, P < 0.001) and elevated AST/ALT (0.73 vs. 0.60, P < 0.001), while other variables showed no significant differences.
| Variables | Overall (N = 327) | Hep C (N = 274) | Hep B (N = 53) | P-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | IQR | Mean | Median | IQR | |||
| Male | 215 (66) | 169 (61.6) | 46 (86.8) | <0.001 b | ||||
| Age (y) | 42 ± 12 | 42 ± 12 | 39 | 20 | 41 ± 13 | 41 | 23 | 0.72 |
| AST/ALT | 0.71 ± 0.26 | 0.73 ± 0.26 | 0.68 | 0.34 | 0.60 ± 0.23 | 0.53 | 0.26 | <0.001 b |
| APRI | 0.95 ± 0.88 | 0.97 ± 0.85 | 0.63 | 0.77 | 0.89 ± 1.01 | 0.58 | 0.60 | 0.47 |
| FIB-4 | 1.61 ± 1.47 | 1.65 ± 1.50 | 1.1 | 1.18 | 1.40 ± 1.27 | 0.92 | 1.18 | 0.35 |
| Forns | 7.03 ± 1.88 | 7.09 ± 1.83 | 6.90 | 2.4 | 6.74 ± 2.12 | 6.46 | 3.04 | 0.18 |
| Fibrosis | 0.13 | |||||||
| 0 | 55 (17) | 43 (16) | 12 (23) | |||||
| 1 | 125 (38) | 111 (41) | 14 (26) | |||||
| 2 | 71 (22) | 60 (22) | 11 (21) | |||||
| 3 | 41 (13) | 30 (11) | 11 (21) | |||||
| 4 | 35 (11) | 30 (11) | 5 (9) | |||||
| Advanced fibrosis | 76 (23) | 60 (21.9) | 16 (30.2) | 0.19 | ||||
a Value are presented as No. (%) or mean ± SD.
b P < 0.05 was considered statistically significant.
All assessed indexes for the whole cohort (N = 327) significantly correlated with liver fibrosis grade (P < 0.001). FIB-4 and the Forns index exhibited a moderate correlation (Spearman’s rho 0.48 for both), while AST/ALT and APRI showed mild correlations (Spearman’s rho 0.21 and 0.31, respectively) (Table 2). In the chronic hepatitis C subgroup (N = 274), FIB-4 and the Forns index had moderate correlations (Spearman’s rho 0.51 and 0.50), while AST/ALT and APRI had mild correlations (Spearman’s rho 0.27 and 0.32) (Table 2). For chronic hepatitis B patients (N = 53), FIB-4 and the Forns index had moderate correlations (Spearman’s rho 0.38 and 0.41), APRI had a mild correlation (Spearman’s rho 0.32), and no correlation existed between AST/ALT and liver fibrosis grade (Spearman’s rho -0.01) (Table 2).
a P < 0.05 was considered statistically significant.
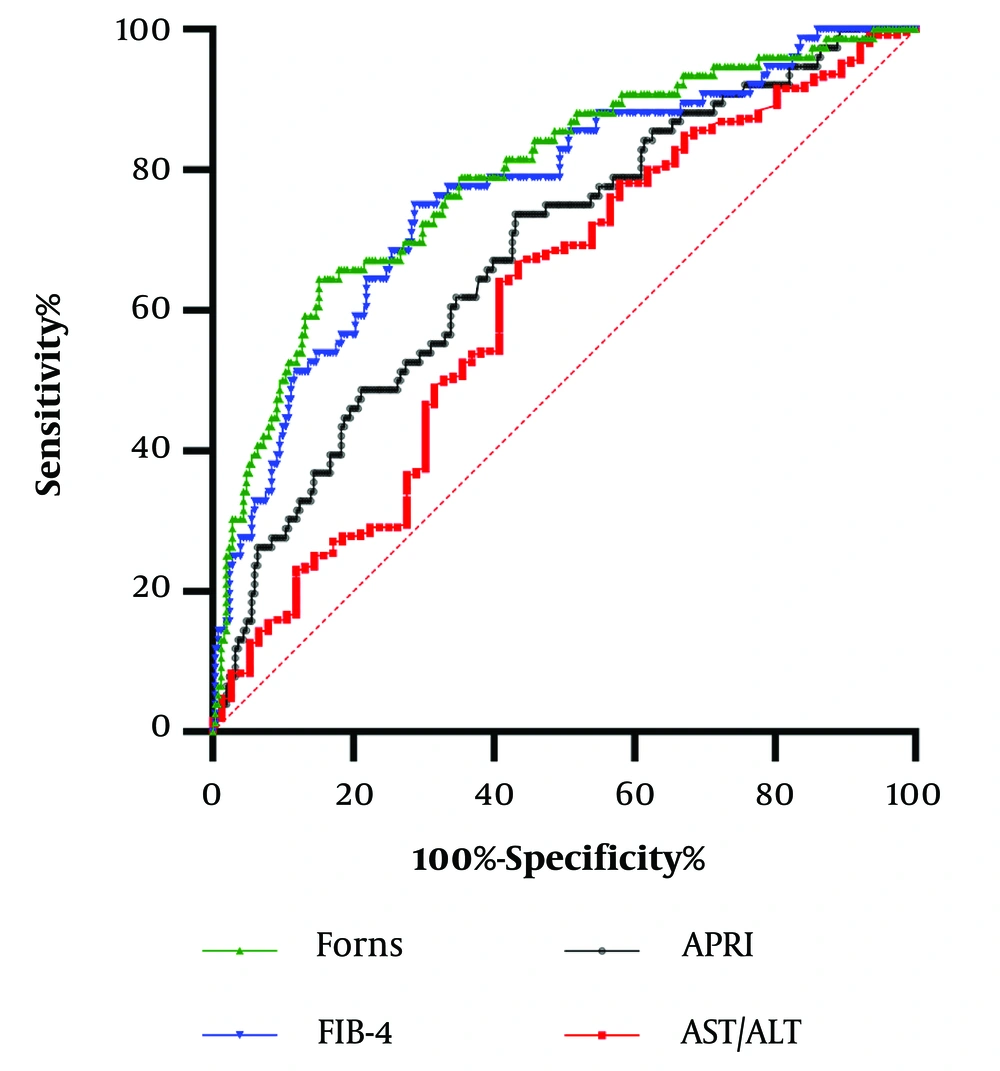
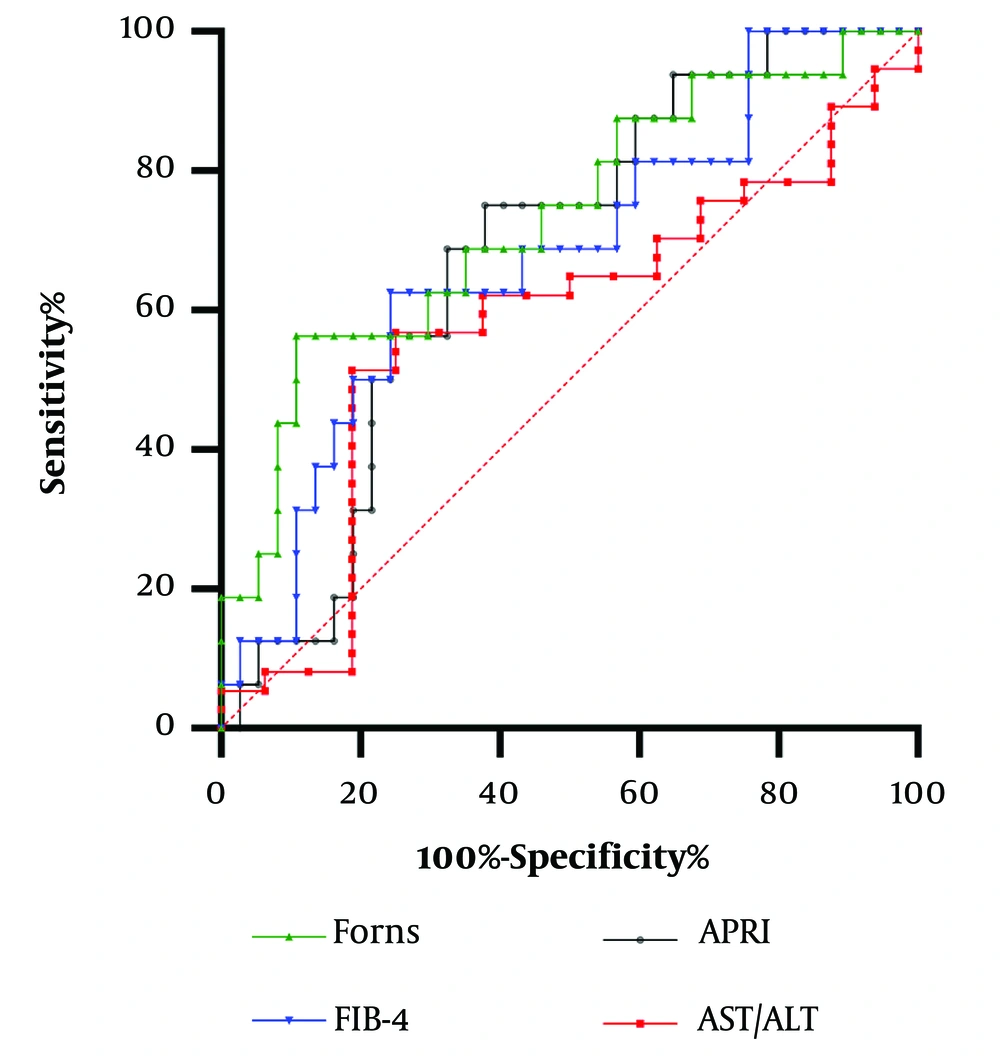
Receiver operator characteristic analysis with AUC calculation were plotted for serological markers for predicting advanced fibrosis in groups of patients with hepatitis C and B.
In the CHC group (N = 274), all of the tested indexes achieved significance in predicting advanced fibrosis (P < 0.001 for all), with the highest AUCs for the Forns index (0.814) and FIB-4 (0.80) being significantly higher than AUCs for APRI (0.69) and AST/ALT (0.68), P < 0.001. The Forns index cutoff of 7.96 indicated 68.3% sensitivity and 82.7% specificity, while the FIB-4 value of 1.46 showed 73.3% sensitivity and 74.3% specificity (Figure 1).
In hepatitis B patients (N = 53), the Forns index (AUC = 0.73), APRI score (AUC = 0.68), and FIB-4 index (AUC = 0.68) had significant predictive ability (P < 0.05 for all), with no difference among them. The Forns index cutoff (8.31) demonstrated 56.3% sensitivity and 89.2% specificity, and the FIB-4 (cutoff 1.41) showed 62.5% sensitivity and 75.7% specificity. The APRI score (cutoff 0.63) indicated 68.8% sensitivity and 67.6% specificity for predicting advanced fibrosis (Figure 2).
5. Discussion
This paper presents novel insights into the non-invasive assessment of liver damage in 327 patients with CHB and CHC. FIB-4 and the Forns index demonstrated the highest statistically significant correlation with liver fibrosis in CHC (P = 0.51; P = 0.50). In predicting advanced fibrosis in hepatitis C, the Forns index (AUC = 0.814) and FIB-4 (AUC = 0.80) achieved the highest AUC, with no significant difference (P = 0.29). Developed in 2002 for untreated CHC patients, the Forns index, based on platelets, gamma-glutamyl transferase, age, and cholesterol, shows a 96% negative predictive value in early stages and a 66% positive predictive value in significant fibrosis (F2-F4) (20, 21). Initially designed for HCV management, the Forns index is now considered a weak fibrosis indicator due to predictive value fluctuations (50 - 85%) (22), potentially influenced by cholesterol variations in genotype 3 HCV, affecting very-low-density lipoprotein metabolism (23, 24).
In contrast to Bukhari et al.'s study suggesting the Forns index as an excellent cirrhosis marker, our sample did not confirm such specificity or sensitivity (22). The Forns index cutoff in our CHC cohort, 7.96, demonstrated 68.3% sensitivity and 82.7% specificity for predicting advanced fibrosis. The reasons, whether related to HCV genotype, cholesterol metabolism impact, patient age, or the use of liver biopsy as our absolute standard, remain to be addressed in future studies. The FIB-4 score, incorporating age, platelet count, AST, and ALT, non-invasively assesses liver condition (25, 26). A value below 1.45 shows a 90% negative predictive value for advanced fibrosis, while above 3.25 exhibits 97% specificity and 65% positive predictive value. Our study's FIB-4 cutoff of 1.46 for advanced fibrosis demonstrated 73.3% sensitivity and 74.3% specificity, aligning with expectations and highlighting its role in tailoring protocols for HCV patients (27-29).
Within our CHC patient group, we noted a weak correlation between liver fibrosis degree and the De Ritis index. An APRI Score exceeding 1.5 signals a higher likelihood of cirrhosis, with 41% sensitivity and 95% specificity for accurate significant fibrosis and cirrhosis prediction (30, 31). The limited APRI Score correlation with fibrosis in our study may stem from intertwined steatosis and toxic hepatitis effects, considering CHC's epidemiological and socioeconomic aspects. This underscores the need for a comprehensive biochemical test panel, such as the FIB-4 algorithm and Forns Index, to identify patients with more severe liver fibrosis accurately. Similarly, to hepatitis C patients, those with CHB displayed the most substantial and statistically significant correlation with liver fibrosis for FIB-4 and the Forns index (P = 0.38; P = 0.41, respectively). Shared mechanisms of fibrinogenesis in chronic viral hepatitis suggest comparable predictive values, specificity, and sensitivity for all serum fibrosis markers (32). In our research, the FIB-4 cutoff (1.41), sensitivity (65%), and specificity (75.7%) showed no significant differences between hepatitis B and C groups.
Similarly to FibroScan examination findings (33), our study identified a slightly higher Forns index cutoff in CHB patients (8.31), demonstrating 56.3% sensitivity and 89.2% specificity for advanced fibrosis compared to CHC patients. Notably, no significant correlation was found between the degree of fibrosis and the De Ritis index, with low correlation observed for the APRI score. This divergence could be attributed to aminotransferase fluctuations during various stages of HBV infection and the influence of underlying liver steatosis (34).
In HBV, age plays a crucial role in fibrosis assessment, corresponding to the duration of infection in Serbia (vertical transmission and transmission in early life) (35). Gender-wise analysis indicated a higher AST/ALT ratio in females with chronic hepatitis (0.75 vs. 0.61, P < 0.001). Our findings align with Amjad et al., who demonstrated higher ALT levels in males with HCV infection aged 21-60 and slightly elevated AST levels in females aged 41 - 60 with hepatitis C (36).
Due to the global prevalence of chronic hepatitis C and/or B, conducting liver biopsy evaluations is impractical, necessitating noninvasive fibrosis assessment. Advanced fibrogenesis can result in progressive architectural distortion, scar tissue formation, and ultimately, liver cirrhosis. The degree of liver fibrosis, whether due to chronic hepatitis C and/or B or other etiologies, is a key prognostic factor, influencing the risk of hepatocellular carcinoma in chronic liver diseases. Extensive research focuses on noninvasive markers for liver fibrosis, not to replace liver biopsy entirely but to restrict its use to specific cases. Noninvasive markers, recommended by the World Health Organization, are preferred over invasive tests. In low- and middle-income countries, APRI and FIB4 are endorsed for their low cost, accessibility, routine use in clinical practice, and accuracy in identifying fibrosis and cirrhosis (37-39).
5.1. Limitations of the Study
Certain limitations of our study should be considered. Since all of the data were obtained from one center, with solely Caucasian patients, it may not be easily extrapolated to different centers due to ethical and racial variations. Furthermore, this pilot study did not include detailed virological parameters of HBV stages (HBe Ag, qHBs Ag, PCR HBV DNA), HCV (genotype, PCR HCV RNA), co-infection with hepatitis D virus, and it is necessary for future research on noninvasive assessment of liver damage to consider these variables. It is important to examine the potential of the investigated serological markers across different cohorts of patients with chronic hepatitis B and/or C. Additionally, conclusions should be drawn regarding whether serological markers can be uniformly used to assess liver damage regardless of virus genotype, co-infection with hepatitis D virus, etc. Given that this is a single-center pilot study with a limited number of participants, we believe it is essential to compare these results with cohorts comprising a larger number of participants, which we plan to achieve through a multicenter study involving a greater number of reference clinical centers in the country.
5.2. Conclusions
Liver fibrosis biomarkers, FIB-4 and the Forns index, offer accurate confirmation or exclusion of advanced liver fibrosis in viral chronic liver disease, aiding in severity assessment. While invasive methods provide precision, these biomarkers serve as crucial non-invasive alternatives, particularly in resource-limited settings. Follow-up studies are vital to explore the predictive nature of these markers in viral chronic liver diseases, particularly in hepatitis B and/or C infections. Our study underscores the importance of FIB-4 and the Forns index as intricate fibrosis management tools in chronic viral hepatitis, with FIB-4 serving as a universal marker and the interpretation of the Forns index requiring consideration of chronic viral hepatitis etiology.