1. Background
Hepatocellular carcinoma (HCC), the most common form of primary liver cancer, ranks among the deadliest malignant tumors affecting humans (1, 2). Major risk factors for HCC include hepatitis B (HBV) and hepatitis C (HCV) infections, excessive alcohol consumption, smoking, obesity, diabetes, and other metabolic disorders (3-5). Recent studies have highlighted that over 40% of new liver cancer cases worldwide are diagnosed in China (6). Despite a noticeable decrease in liver cancer incidence, China still bears the highest burden of liver cancer globally (2, 6).
Oxidative stress has been implicated in various chronic liver diseases and contributes to the development of hepatocarcinogenesis (7, 8). Oxidative stress occurs due to an imbalance between the generation and clearance of superoxide radicals (O2-) in the body. Superoxide dismutases (SODs) are a group of metalloenzymes that catalyze the dismutation of O2- into molecular oxygen (O2) and hydrogen peroxide (H2O2) (9, 10). Thus, SOD serves as an indicator for assessing oxidative stress due to its pivotal role in the cellular antioxidant defense system. Humans have three isoforms of SOD, each with distinct localizations: Homodimeric copper (Cu)/zinc (Zn)-SOD (SOD1) is expressed in the cytoplasm, manganese (Mn)-SOD (SOD2) is located in the mitochondrial matrix, and iron (Fe)-SOD (SOD3) is found in the extracellular matrix (11). Given that SOD is a metalloenzyme, maintaining appropriate concentrations of trace metal elements is crucial for its activity (12). Recent studies have also indicated that selenium (Se) can enhance SOD activity (13, 14).
Indeed, SOD has a dual effect, as the product H2O2 can react with ferrous iron to produce cytotoxic hydroxyl radicals (8, 11). Therefore, data regarding SOD in tumors are ambiguous: On one hand, it acts as a tumor suppressor and can enhance cancer treatment; on the other hand, it promotes the invasive and migratory activity of tumor cells (11, 15-17). Regarding HCC, previous studies have primarily focused on the expression and activity of SOD in blood and HCC cells (18-21).
2. Objectives
The objectives of this cohort study were as follows: (1) to assess changes in serum SOD activity in HCC patients; (2) to determine the diagnostic cutoff value of SOD in HCC; (3) to explore the association between serum SOD activity and all-cause mortality in HCC patients; (4) to analyze the associations between whole blood trace element concentrations and SOD activity in HCC patients.
3. Methods
3.1. Study Design and Ethics
The retrospective case-control study of the SOD Model was conducted using a single-center dataset from Shandong Provincial Hospital Affiliated with Shandong First Medical University, China. Hepatocellular carcinoma patients included in the model group were diagnosed in the early stages between 2012 and 2014 and were followed up. This study was approved by the Medical Ethics Committee of Shandong First Medical University in compliance with the Declaration of Helsinki (ethical approval number: NSFC: No.2021-373).
3.2. Patient Selection
A total of 241 participants were screened between 2011 and 2022 for the study. Clinical data of 124 HCC patients (94 cases diagnosed between 2012 and 2014 in the model group and 30 cases diagnosed in 2022 in the validation group) were obtained from the Liver and Gallbladder Surgery Department's database. The clinical staging of HCC patients ranged from Ia ~ IIa according to the China Liver Cancer Staging (CNLC), and the child-Pugh classifications were A to B. Inclusion criteria were as follows: Patients histologically diagnosed with HCC according to the Edmondson-Steiner classification system (22). Exclusion criteria were as follows: Presence of other metastatic liver tumors or prior oncological therapy for any malignant disease before surgery.
A total of 117 healthy subjects (87 cases from 2021 were included in the model group, and 30 cases from 2022 were included in the validation group) were recruited from the health examination center. Inclusion criteria were as follows: No complaints of discomfort, no abnormalities in physical examination, and no other acute or chronic diseases. Exclusion criteria were as follows: Diagnosis of tumors, and suffering from acute or chronic diseases of other systems.
3.3. Data Collection
The medical reports of tumor size, tumor number, differentiation grade, and vascular invasion of the HCC patients were obtained from the Pathology Information System. Diagnosis was histologically confirmed in all cases, primarily based on the examination of sections stained with H&E. Other serological biomarkers of the participants were assessed during routine clinical procedures before operation. Additionally, medical reports of SOD activity (for the model group), alpha fetoprotein (AFP), and alanine aminotransferase (ALT) levels were obtained from the laboratory information system.
3.4. SOD Activity and Trace Element Assay
The serum of the participants in the validation group was stored at -80°C for the assessment of SOD activity and trace elements. The analytical methodology, reagents, and equipment used for detecting SOD activity remained consistent with those used in the model group.
The methods used for serum total SOD activity analysis were as follows: Serum samples were analyzed using the Olympus AU58 series automatic biochemical analyzer under the following conditions: Determination wavelength of 420 nm, endpoint method as the reaction method, and a reaction temperature of 37°C. The detection reaction principle employed was the Pyorgallol autoxidation method (PAM). Single-point linear calibration products and dual-level quality control (QC) products were manufactured by Fujian Fuyuan Biotechnology Co., LTD. The intra-assay precision CVs were 4.02% for lower QC and 1.10% for higher QC, while the inter-assay precision CVs were 3.47% for lower QC and 2.41% for higher QC, respectively. The accuracy was validated through comparative experiments, with a deviation of less than 10%.
The remaining serum after measuring SOD activity was utilized for elemental content analysis. Mn, Fe, Cu, Zn, and Se were detected using the limiting dilution method with agilent 7900 inductively coupled plasma mass spectrometry (ICP-MS) (Agilent, Tokyo, Japan). Ultrapure water (≥ 18.0 MΩ.cm, 25°C) used in the experiment was prepared by a Milli-Q ultrapure water meter (Millipore Corporation, USA). Serum samples were pre-diluted ten times with a solution containing Au in 0.5% nitric acid and 0.01% Triton X-100. The setting parameters for the ICP-MS method are shown in Appendix 1. The internal standard recovery was within 80 ~ 120%. The linear r values of all the elements' calibration curves were over 0.999. For quality assurance, accuracy was evaluated by recovery (all deviations were < 15%). Low and high value QCs were performed at the start and end of each detection.
3.5. Statistical Analysis
Statistical analyses were performed using GraphPad Prism software version 9.0 (GraphPad, La Jolla, CA, USA). The Shapiro-Wilk normality test was used for normality testing. Normally distributed variables were presented as mean ± standard deviation (SD), while abnormally distributed variables were presented as median (Q1, Q3). The clinicopathological parameters of the H-SOD group were compared with those of the L-SOD group. Univariate analyses between two groups were performed using Student’s t-test and the Mann-Whitney U test for continuous variables, or the chi-square test for noncontinuous variables. The comparison of constituent ratios between two groups was assessed by the chi-square test or Fisher's test. Receiver operating characteristic (ROC) curves, areas under the ROC curves, and 95% confidence intervals for area under the curves (AUCs), along with Youden’s index, were calculated to determine cutoff values of serum total SOD activity for the diagnosis of HCC. Sensitivity and specificity were calculated for the standard cutoff. Overall survival (OS) was determined via the Kaplan-Meier method. Spearman or Pearson coefficient values for correlation of two statistical variables were also determined. All P-values were two-tailed, and P-values less than 0.05 were considered significant.
4. Results
4.1. Baseline Characteristics of the Study Group
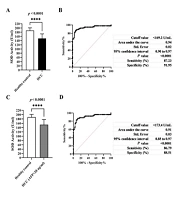
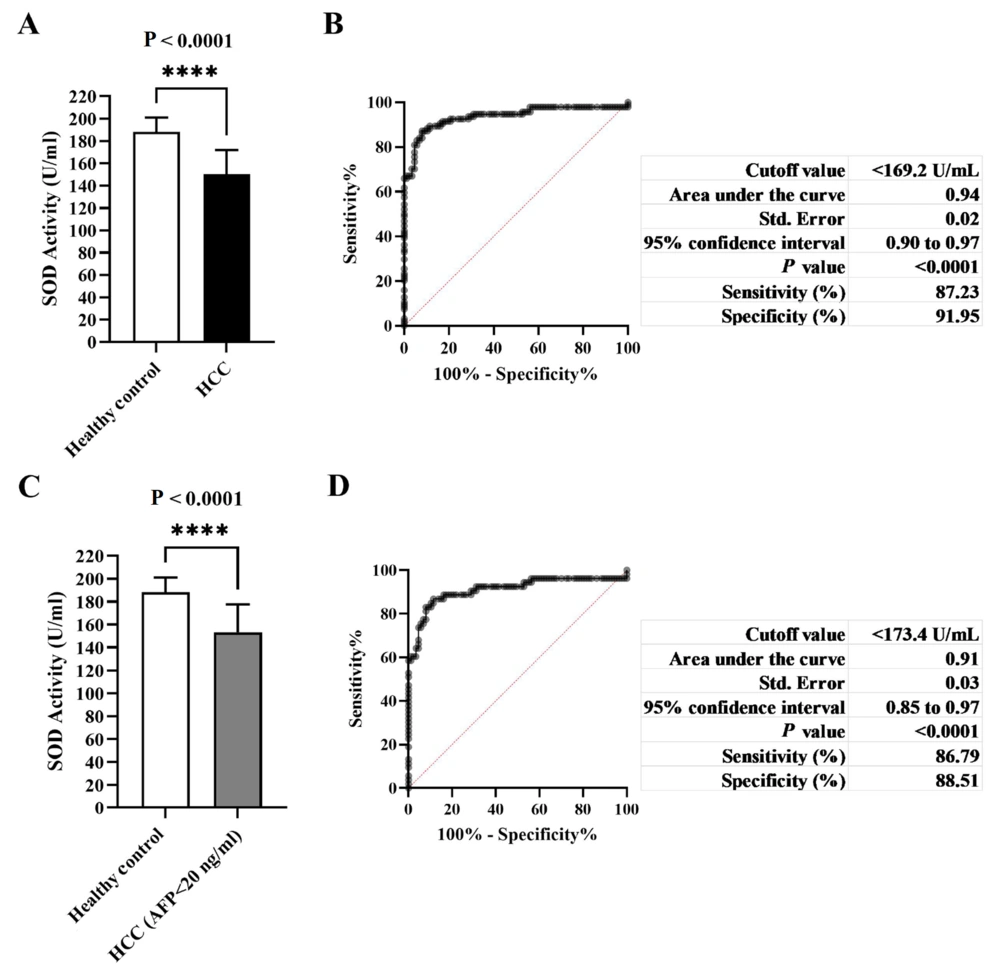
A total of 124 patients with HCC and 117 healthy subjects were included in this single-center retrospective study in China. The baseline characteristics of the model groups and the validation groups are presented in Table 1. No significant differences were observed in age and sex distribution between HCC patients and control subjects in the model groups and the validation groups. Differences in the values of biochemical parameters were also noted. The median serum ALT was significantly higher in HCC patients compared to control subjects in the model groups and the validation groups, respectively. Serum AFP concentration was significantly higher in HCC patients than in control subjects in the model groups, the model groups (AFP < 20 ng/mL), and the validation groups. Furthermore, HCC patients exhibited a predominant reduction in total SOD activity compared to control subjects in the four groups.
| Parameter | Model Groups | Model Groups (AFP < 20 ng/mL) | Validation Groups | Validation Groups (AFP < 20 ng/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCC (N = 94) | Control (N = 87) | P-Value | HCC (N = 53) | Control (N = 87) | P-Value | HCC (N = 30) | Control (N = 30) | p-Value | HCC (N = 18) | Control (N = 30) | P-Value | |
| Age (y) | 55.8 ± 9.8 | 55.9 ± 10.1 | 0.9598 | 58.3 ± 9.2 | 55.9 ± 10.1 | 0.1584 | 57.5 ± 10.1 | 54.7 ± 10.6 | 0.3042 | 58.8 ± 11.3 | 54.7 ± 10.6 | 0.2073 |
| Sex (M/F) | 84/10 | 75/12 | 0.6499 | 49/4 | 75/12 | 0.4118 | 26/4 | 23/7 | 0.5062 | 15/3 | 23/7 | 0.7222 |
| ALT (U/L) | 33 (22 - 55) | 21 (16 - 26) | < 0.0001 | 30 (20 - 49.5) | 21 (16 - 26) | 0.059 | 33.5 (17 - 56.3) | 16 (13 - 20.3) | < 0.0001 | 25 (16 - 37) | 16 (13 - 20.3) | 0.0019 |
| AFP (ng/mL) | 12.9 (3.4-594.3) | 2.6 (1.8 - 3.9) | < 0.0001 | 3.7 (2.3 - 8.5) | 2.6 (1.8 - 3.9) | 0.0026 | 7.3 (1.8 - 267.9) | 2.7 (1.9 - 4.4) | 0.0106 | 2.2 (1.5 - 5.8) | 2.7 (1.9 - 4.4) | 0.8619 |
| SOD (U/mL) | 151.5 (135.0 - 161.3) | 189.6 (179.3 - 196.3) | < 0.0001 | 154 (132.5 - 166.0) | 189.6 (179.3 - 196.3) | < 0.0001 | 134.6 ± 21.6 | 189.7 ± 12.1 | < 0.0001 | 134.9 ± 23.9 | 189.7 ± 12.1 | < 0.0001 |
Baseline Characteristics and Superoxide Dismutase Levels of Hepatocellular Carcinoma Patients and the Healthy Control Group for Model and Validation a
4.2. Cutoff Points of Serum Total SOD Activity for the Diagnosis of HCC Using ROC Curves
The average serum total SOD activity in HCC patients was significantly reduced compared to healthy controls (Table 1, Figure 1A - C). The optimal SOD activity cutoff level for defining HCC in the model group was determined at 169.2 U/mL using ROC curves, with an AUC of 0.94 (Figure 1B). Furthermore, in the validation group, we found that the true positive rate was 93.33%, the true negative rate was 96.67%, and the accuracy rate was 95.00%. Similarly, the optimal SOD activity cutoff level for defining HCC (AFP < 20 ng/mL) in the model groups was determined at 173.4 U/mL, with an AUC of 0.91 (Figure 1D). In the validation group, the true positive rate was 94.44%, the true negative rate was 93.33%, and the accuracy rate was 93.75%. These results indicate that the serum SOD activity cutoff value was established and could accurately identify HCC.
Histograms, receiver operating characteristic (ROC) curve as well as cutoff values, areas under the ROC curve (95% CI), sensitivity and specificity for parameters differentiating hepatocellular carcinoma (HCC) patients vs. the control group (A and B); HCC patients with alpha fetoprotein (AFP) < 20 ng/mL vs. the control group (C and D).
4.3. The Predictive Sensitivity and Accuracy of Serum SOD Activity in HCC Appears to Be Better Compared with AFP
Our analysis indicated that serum SOD activity serves as a good prognostic parameter in HCC (Table 2). The diagnostic sensitivity of serum SOD activity is much higher than that of AFP (43.62% vs. 87.23%, P < 0.0001). Similar results were obtained for diagnostic accuracy (63.95% vs. 89.50%, P < 0.0001).
The Frequency of Sensitivity, Specificity and Accuracy of Alpha Fetoprotein and Superoxide Dismutase in Diagnosis of Hepatocellular Carcinoma
4.4. Clinicopathological Features of H-SOD and L-SOD Patients with HCC
Table 3 presents the clinicopathological characteristics of patients with HCC classified based on total SOD activity into H-SOD (total SOD activity > 169.2 U/mL) and L-SOD (total SOD activity < 169.2 U/mL) groups. No obvious differences in baseline parameters were observed between the two groups.
| Parameters | HCC | P-Value | |
|---|---|---|---|
| H-SOD (n = 12) | L-SOD (n = 82) | ||
| Epidemiology | |||
| Age | 55.0 ± 13.0 | 55.9 ± 9.3 | 0.7635 |
| Sex (M/F) | 12/0 | 72/10 | 0.3509 |
| Underlying liver disease | |||
| Alcohol | 1 | 4 | 0.5029 |
| HBV | 8 | 69 | 0.2200 |
| HCV | 0 | 2 | > 0.9999 |
| NASH | 1 | 1 | 0.2402 |
| Others | 2 | 6 | 0.2700 |
| Biochemical factors | |||
| ALT | 30 (17.5 - 42.5) | 33 (23 - 55.5) | 0.2362 |
| AFP (ng/mL) | 4.9 (3.8 - 329.2) | 13.6 (3.1 - 643.1) | 0.6389 |
| Therapy for HCC | |||
| Resection | 12 | 82 | > 0.9999 |
| Resection + TACE | 3 | 16 | 0.7032 |
| Resection + locoregional ablation | 1 | 3 | 0.4264 |
| Tumor factors | |||
| Tumor size (cm) | 0.538 | ||
| > 5 | 4 | 38 | |
| ≤ 5 | 8 | 44 | |
| Tumor number | 0.2023 | ||
| Single | 12 | 68 | |
| Multiple | 0 | 14 | |
| Histological factors | |||
| Differentiation: III | 1 | 14 | 0.6832 |
| Fibrosis | 5 | 42 | 0.7586 |
| Vascular invasion | 4 | 34 | 0.7562 |
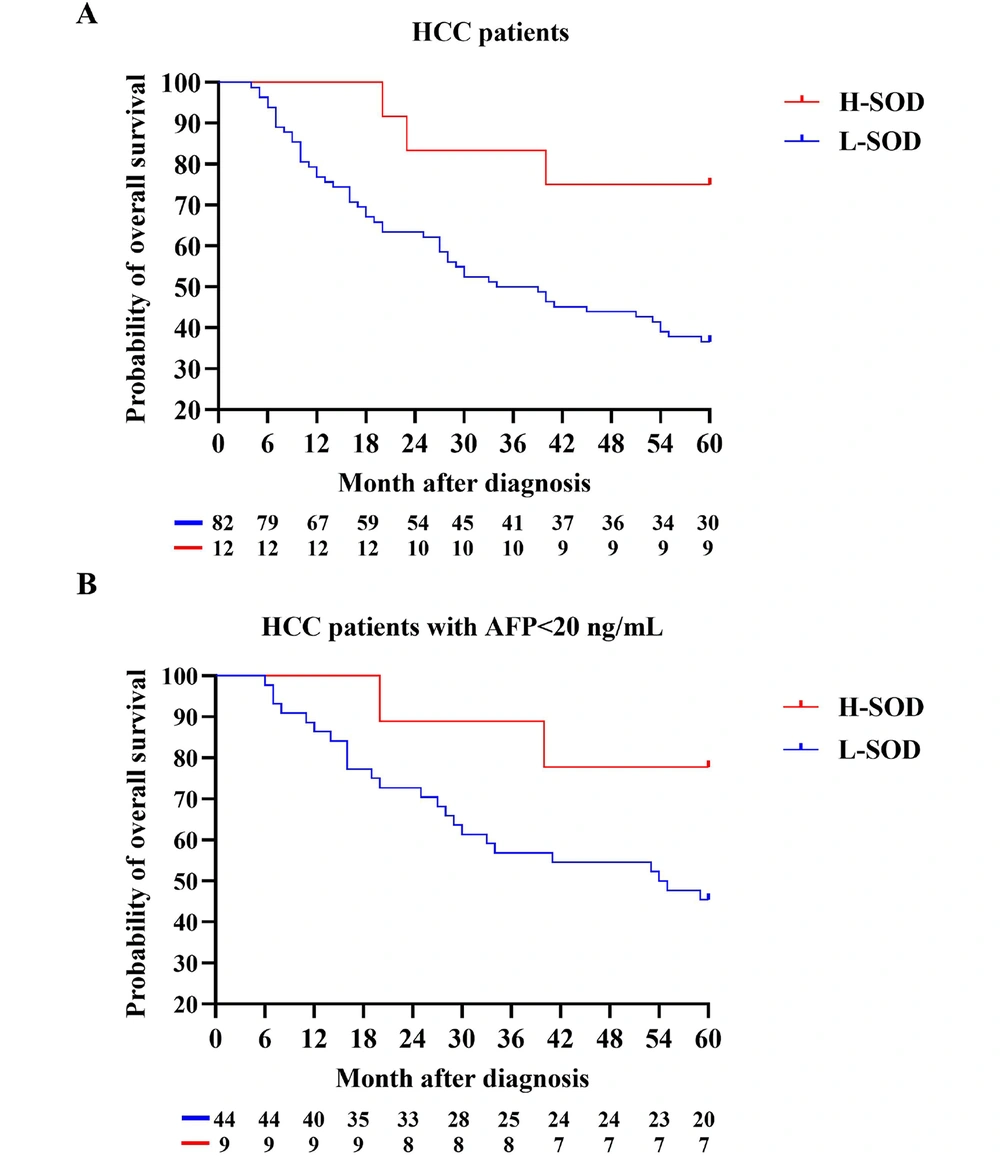
4.5. Prognoses Based on SOD Activity in Patients with HCC
Kaplan-Meier OS estimates according to the cutoff value of serum total SOD activity before operation are presented in Figures 1 and 2. Serum SOD activity below and above the cutoff value significantly affected the probability of 5-year OS in HCC patients (Figure 2 and Table 4).
| Parameters and Model | H-SOD (%) | L-SOD (%) | P-Value |
|---|---|---|---|
| 2-year OS | |||
| HCC | 83.33 | 65.85 | 0.3265 |
| HCC (AFP < 20 ng/mL) | 88.89 | 75.00 | 0.6652 |
| 5-year OS | |||
| HCC | 75.00 | 36.59 | 0.0245 b |
| HCC (AFP < 20 ng/mL) | 77.78 | 45.45 | 0.1415 |
Comparison of H-Superoxide Dismutase and L-Superoxide Dismutase in prediction of 2-year Overall Survival and 5-year Overall Survival a
4.6. Decrease in SOD Activity in HCC Patients Related to Specific Trace Elements
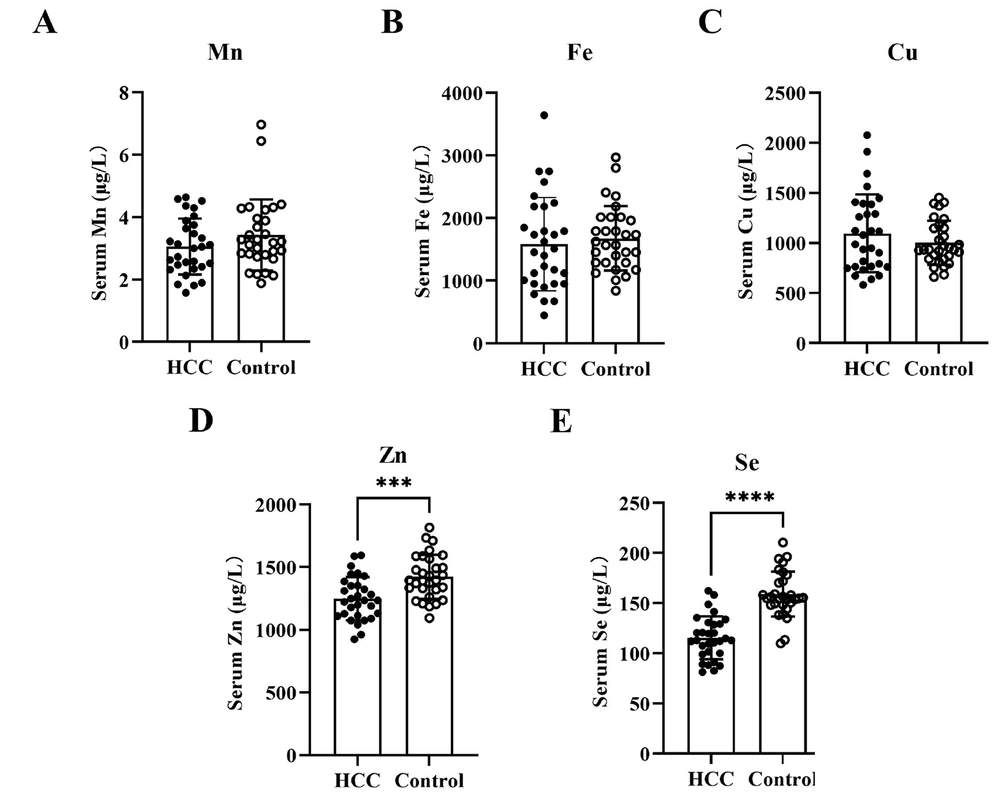
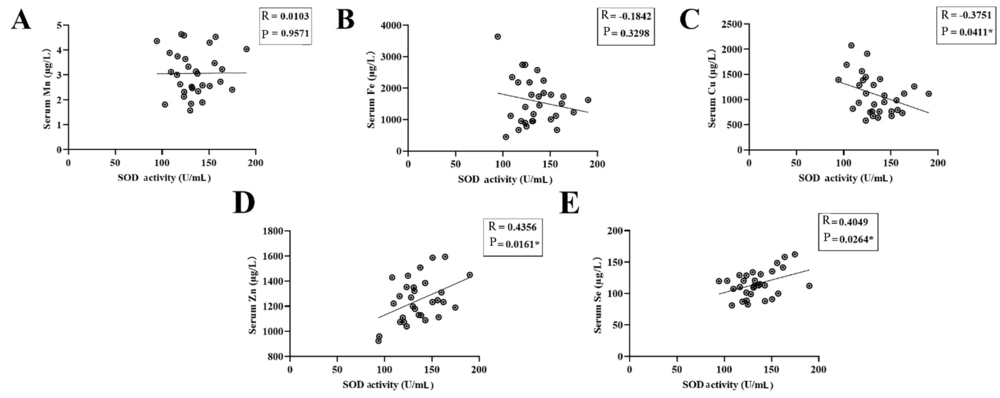
As a metalloenzyme, SOD activity is closely related to trace metal elements. The concentrations of serum Mn, Fe, Cu, Zn, and Se in the validation groups were assayed. Accompanied by a decrease in SOD activity, the serum levels of Zn (t = 3.890, P = 0.0003) and Se (t = 7.694, P < 0.0001) were also significantly decreased in HCC patients (Figure 3D, E). Compared with the control group, the serum levels of Mn, Fe, and Cu in HCC patients did not change significantly (Figure 3A-C). Accordingly, Pearson's test revealed positive correlations between Se/Zn concentration and SOD activity in patients with HCC (r = 0.4049/r = 0.4356, both P < 0.05) (Figure 4D, E). Conversely, Spearman's test revealed a negative correlation between Cu concentration and SOD activity in patients with HCC (r = -0.3751, P < 0.05) (Figure 4C). No correlation between Mn/Fe concentration and SOD activity was observed in patients with HCC (r = 0.0103/r = 0.1842, P > 0.05) (Figure 4A, B).
Comparison of trace elements contents (µg/L) in hepatocellular carcinoma (HCC) patients and healthy subjects. A, B, and C, no obvious difference of serum Mn, Fe and Cu was observed between HCC patients and healthy subjects; D and E, the levels of serum Zn and Se in HCC patients were obviously lower than that in healthy subjects (*** P < 0.001, **** P < 0.0001).
The correlation between serum superoxide dismutase (SOD) activity and the contents of various trace elements in hepatocellular carcinoma (HCC). A and B, no significant correlation of Mn or Fe level and serum SOD activity; C, negative correlation of Cu level and serum SOD activity; D and E, positive correlations of Zn or Se level and serum SOD activity (* P < 0.05).
5. Discussion
In this study, we illustrated the significance of Zn/Se-dependent SOD activity as a diagnostic and predictive marker for HCC. Compared to AFP, the most commonly used serum biomarker for detecting HCC, serum SOD activity showed markedly improved sensitivity and accuracy. Furthermore, SOD emerged as a reliable biomarker for diagnosing AFP-negative HCC.
Previous studies mainly focused on SOD activity and concentrations within HCC tumor tissues (18, 19, 23). Similarly, we observed decreased serum SOD activities in HCC patients. Serum, being a readily available biological material from suspected HCC patients, offers convenience and accessibility. Moreover, detecting serum SOD activity is less invasive and more cost-efficient compared to obtaining tissue sections. Therefore, the clinical utility of serum SOD activity appears to surpass that of tissue-based analysis.
Alpha fetoprotein is widely employed as a serum biomarker for HCC screening. However, many cases of HCC do not exhibit elevated AFP levels. The sensitivity of AFP alone in diagnosing HCC is only around 43.62%, consistent with our findings (24-26). Currently, various studies are underway to identify reliable biomarkers for diagnosing AFP-negative HCC (27). Biomarkers such as AFP-L3, PIVKA-II, and MicroRNAs have been explored for this purpose. Our study highlights the promising value of serum SOD activity in detecting AFP-negative HCC. Hence, individuals with predisposing factors for HCC should remain vigilant for early HCC development, especially if serum SOD activity decreases (20, 21).
The development and progression of HCC are accompanied by persistent oxidative stress, although the underlying mechanism is still not well defined. Studies on SOD1 (Cu, Zn-SOD) knockout mice have reported the induction of hepatic collagen accumulation, leading to the development of liver fibrosis (28). Conversely, SOD2 (Mn-SOD) acts as a tumor suppressor, and its expression is often reduced in many cancers. The significance of Mn-SOD in tumor suppression and its potential to enhance cancer treatment may be related to its bidirectional regulation with P53 (19, 29). While our study measured total SOD activity without distinguishing between SOD1 and SOD2, we also analyzed the metal content in HCC patients. Since SOD is a metalloenzyme that requires a metal cofactor for its activity, we assessed the levels of Zn and Se elements, which were found to be positively correlated with serum SOD activity but decreased in HCC patients. Interestingly, we did not observe a reduction in Mn levels in HCC, contrary to findings from previous studies (15, 16). Although Cu is a metallic component of SOD1, we found a negative correlation between serum Cu and SOD activity in HCC patients, suggesting that Cu may not enhance SOD activity in this context. Based on our findings, we recommend that HCC patients pay special attention to the supplementation of Zn and Se elements.
Tamai et al. also reported that serum Mn-SOD levels are potential clinical biomarkers predicting patient prognosis in HCV-related HCC (19). Importantly, our study not only determined the diagnostic cutoff value of SOD in HCC using clinical samples but also observed the relationship between SOD and OS. All HCC patients in this study underwent one-stage hepatectomy. Subsequently, transcatheter arterial chemoembolization (TACE) or locoregional ablation were performed in 23 out of the 94 HCC patients. None of the patients received targeted, immune, or other advanced treatments. As expected, higher serum SOD activity in HCC patients before operation significantly increased the probability of 5-year OS. Although in HCC patients with AFP < 20 ng/mL, serum SOD activity below the cutoff value decreased the probability of 5-year OS from 77.78% to 45.45%, the small sample size precluded statistical significance. With the application of TACE, hepatic arterial infusion chemotherapy (HAIC), and targeted immune quadruple therapy for patients with intermediate and advanced-stage HCC (30), we can further observe the early warning effect of serum SOD activity in combination immunotherapy for advanced HCC.
The limitations of this single-center study in China include its small sample size and the lack of postoperative tracking of SOD activity in patients. However, we demonstrated that serum SOD activity outperforms the application of AFP alone for HCC detection. It is worth mentioning that SOD activity is closely related to age, which should be considered in HCC diagnosis. This relationship may lead to increased false-positive results and limit the specificity of SOD.
Hepatocellular carcinoma has a high incidence and low five-year survival rate in China. Effective therapeutic protocols for unresectable patients with advanced HCC are lacking. Early diagnosis and surgery remain the main options for HCC treatment. It is suggested that patients susceptible to HCC should monitor SOD activity to assess the degree of oxidative stress in the early stages and predict the prognosis of HCC.